Nuclear Fusion is the name given to the the event when the nuclei of two atoms combine creating a new heavier atomic nucleus.
What is Nuclear Fusion?
Nuclear Fusion is a phenomenon where by the nucleus of two atoms fuse together creating a new heavier atomic nucleus. When two relatively small atomic nuclei have very high energies they are able to bond or fuse together making larger atoms. The atomic nuclei require a lot of energy to overcome the effects of the electromagnetic force before it is possible for nuclear fusion to occur.
Overcoming the Electromagnetic Force
Normally the electromagnetic force causes atomic nuclei to repel each other due to their positive charge, much like the same poles on a magnet. Electromagnetic force which causes the repulsive action quadruples as the distance between the two protons halves. This means that it takes a lot of energy to get these two protons, and two nuclei together. If they are able to get close enough another fundamental force, The Strong Force, comes into play and this force is much stronger than the electromagnetic force. It is the force that holds all nucleons together but they must be within one femtometres (the nucleus of an average atom is 4 femtometres in diameter).
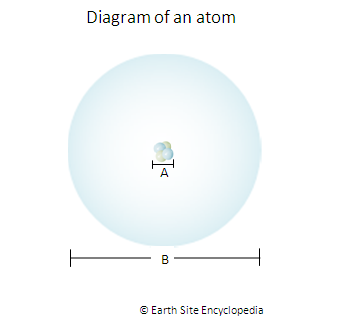
A = The Nucleus of the atom contains the protons and neutrons. Despite accounting for the majority of an atom’s mass, the nucleus procures a minute proportion of the total space. The Diameter of the nucleus is approximately 4 femtometres or 4 x 10 -15 meters. It is very difficult to measure the diameter of the nucleus because like our atmosphere it has no defined edge but instead gradually fades away.
B = this is mainly just empty space where the electrons orbit the nucleus. Its diameter (and that of the whole atom) is 0.1 nanometres or 0.1 x 10-9 meters.
Nuclear Fusion in Stars
This occurs in our sun where the temperature is in excess of 14 million degrees Kelvin turning the hydrogen atoms into plasma. When two hydrogen atoms have immense energy in the forms of heat and pressure, they fuse together to produce a deuterium atom which is a stable isotope of hydrogen. The process involves one of the protons from the hydrogen atoms changing into a neutron and releasing a positron (an electron with a positive charge) and a neutrino.
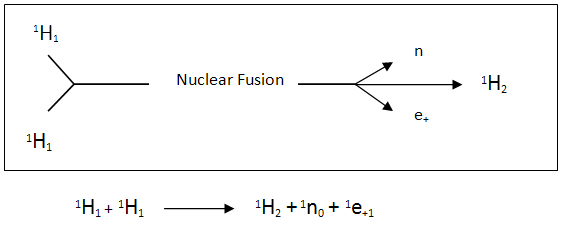
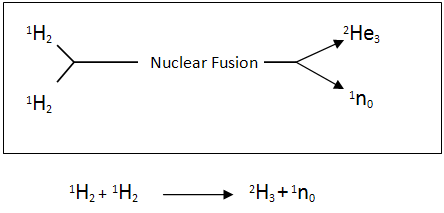
Two of these deuterium atoms can fuse together to produce a helium-3 atom releasing a neutron.
Energy Production through Nuclear Fusion

When the atoms fuse together in the sun their combined mass is slightly less than that of its separate parts.
For example a Hydrogen atom has an atomic mass of 1.00794. If two combined to produce a Deuterium atom you should expect its atomic mass to be about 2.01588 but what we find is that Deuterium actually has an atomic mass of 2.014102. This may not sound like a lot but it is a difference in mass of 0.001778u and even taking away the minute mass of an electron (0.00054u) we have a lost mass of 0.001238u.
Einstein’s famous equation ‘E=Mc2’ shows that mass and energy are interchangeable and this is exactly what happens to the lost mass. Every time atoms are fused together mass is lost and converted into energy. In fact 1 gram of Deuterium can produce 275, million kcal (1012 joules) of energy which is more than enough to heat the water of 3,430 average household baths (275,000 litres) by 1 degree centigrade. In comparison the most that can be produced through fossil fuels is 10 kcal per gram of fuel which would only heat 10ml of water by 1 degree centigrade.
Nuclear fusion is the dream of many scientists as a new energy source for mankind but it requires huge amounts of energy to start the process. In fact all attempts to produce enough energy to ignite fusion have proved inadequate.