Isotopes are the variations of elements. An element is defined by the amount of its protons while an isotope of that element has different amount of neutrons.
Unstable Isotopes (Radioactive Isotopes)
Alpha Radiation or Alpha Decay
What are Isotopes?
 Here is the chemical symbol for helium as you might see it on a periodic table. The number at the top is known as the atomic number and is actually the number of protons that are found in the atom. The number at the bottom is the atomic mass of the element. As electrons are considered to little or no mass the atomic weight is the amount of protons and neutrons normally found in that element. While the atomic number of an element will always remain the same (hydrogen will always have one proton, helium will always have two etc) the atomic mass can change naturally. This happens when more neutrons are added to the element in some way.
Here is the chemical symbol for helium as you might see it on a periodic table. The number at the top is known as the atomic number and is actually the number of protons that are found in the atom. The number at the bottom is the atomic mass of the element. As electrons are considered to little or no mass the atomic weight is the amount of protons and neutrons normally found in that element. While the atomic number of an element will always remain the same (hydrogen will always have one proton, helium will always have two etc) the atomic mass can change naturally. This happens when more neutrons are added to the element in some way.
How are isotopes formed?
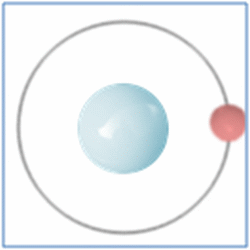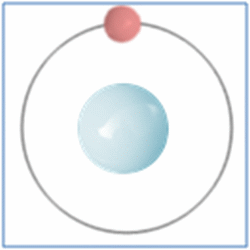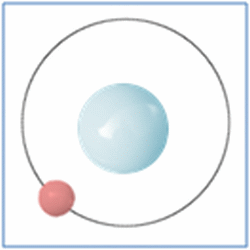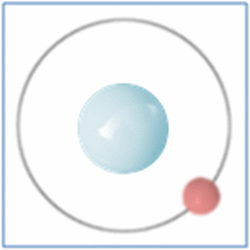 Taking the most basic element, hydrogen, it has a one proton and one electron as shown in the diagram. In the process of nuclear fusion in the sun these hydrogen atoms are split into individual elements of protons and electrons that float around in the form of plasma. The pressure and heat that cause the hydrogen to turn to plasma, rather than gas, also make it easy for the particles to form new atoms. When one of these free protons collides with a hydrogen atom they combine so you have one electron and two nucleons (protons and neutrons are known as nucleons) in the centre. As two protons (which are both positively) would unbalance the single electron (which is negatively charged) one of the protons changes into a neutron.
Taking the most basic element, hydrogen, it has a one proton and one electron as shown in the diagram. In the process of nuclear fusion in the sun these hydrogen atoms are split into individual elements of protons and electrons that float around in the form of plasma. The pressure and heat that cause the hydrogen to turn to plasma, rather than gas, also make it easy for the particles to form new atoms. When one of these free protons collides with a hydrogen atom they combine so you have one electron and two nucleons (protons and neutrons are known as nucleons) in the centre. As two protons (which are both positively) would unbalance the single electron (which is negatively charged) one of the protons changes into a neutron.
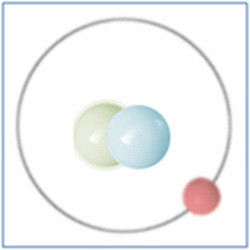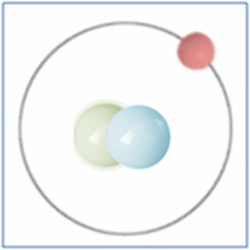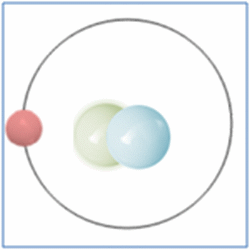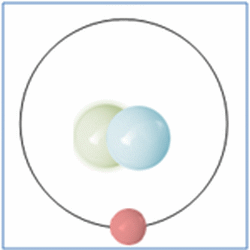 Now the atom has one positively charged particle, the proton, one negatively charged particle, the electron, and one neutral particle the neutron. This makes the particle balanced and complete. The atom is still a form of hydrogen as it has only one proton, yet it is twice the mass of the common form of hydrogen as it has two nucleons (which form nearly all the mass of atoms). This is known as an isotope and this particular isotope of hydrogen is known as Deuterium. Deuterium is sometimes known as Heavy-Hydrogen (as found in heavy water) and can be written as 2H. Deuterium is much rarer than ‘normal’ hydrogen but sometimes hydrogen can be found in another form or isotope known as Tritium which is much rarer still and can be written as 3H. Many of the elements can be found in these ‘heavier’ forms and are naturally occurring. Some of these isotopes such as Carbon-14 are formed by cosmic rays in the upper atmosphere that denature the more common forms of the elements found there.
Now the atom has one positively charged particle, the proton, one negatively charged particle, the electron, and one neutral particle the neutron. This makes the particle balanced and complete. The atom is still a form of hydrogen as it has only one proton, yet it is twice the mass of the common form of hydrogen as it has two nucleons (which form nearly all the mass of atoms). This is known as an isotope and this particular isotope of hydrogen is known as Deuterium. Deuterium is sometimes known as Heavy-Hydrogen (as found in heavy water) and can be written as 2H. Deuterium is much rarer than ‘normal’ hydrogen but sometimes hydrogen can be found in another form or isotope known as Tritium which is much rarer still and can be written as 3H. Many of the elements can be found in these ‘heavier’ forms and are naturally occurring. Some of these isotopes such as Carbon-14 are formed by cosmic rays in the upper atmosphere that denature the more common forms of the elements found there.
Simply put an isotope of an element contains a different amount of neutrons. Hydrogen has three isotopes; hydrogen, deuterium and tritium.
If an element gains an extra proton through a chemical/nuclear reaction it becomes a new element; for example when two Deuterium atoms combine, or fuse together, they become a helium atom.
Stable Isotopes
Some of these isotopes are what is called ‘stable’ and this means that they remain as they are and do not change naturally into their lighter form without an additional force (Deuterium atoms are transformed into helium atoms through nuclear fusion in the sun). In the natural world there are normally more stable isotopes than unstable isotopes.
Unstable Isotope (Radioavtive Isotopes)
These are isotopes that are not stable and attempt to change into a more stable isotope or the basic element. These unstable isotopes change naturally by emitting or throwing off part of the atom. This is known as radioactive decay and occurs in one of two ways.
Alpha Radiation or Alpha Decay
An unstable isotope may give off or emit an “alpha particle” made up of 2 protons and two neutrons (or a helium particle) in order to become more stable. For example the most common isotope on earth is Uranium-238 and it will emit a helium particle to become Thorium-234 as shown below.
92U238 = 2He4 + 90Th234
However Thorium-234 is in itself an unstable isotope and this too decays into a slightly more stable isotope. Uranium-238 goes through a long process of radioactive decay before it becomes fully stable and this process is known as a “Decay Chain”. You can see the entire process or decay chain of Uranium-238 here.
Beta Radiation or Beta Decay
An unstable isotope may give off or emit an electron particle in order to become more stable. As the electron leaves the atom the charge need to be balanced and so one of the protons becomes a neutron. For example the carbon isotope known as Carbon14 (14C) and will emit an electron and become the stable element of Nitrogen as shown below.
6C14 = 7N14 + 0e-1
Radioactive half-life
It is impossible to predict when an single atom in a sample will go through its radioactive decay process however the rate of radioactive decay in any sample will decrease at steady rates. For example a sample of Uranium-238 will be half as radioactive after 4.468 billion years where as a sample of Uranium-234 will take 247,000 years for its rate of decay to decrease by half. This time frame is known as the radioactive half-life and it plays a very important roll in allowing scientist to measure long periods of time. See Radioactive Dating