The Standard model of particle physics (or quantum field theory) is the ultimate theory of everything. The standard model explains interactions between all subatomic particles and the fundamental forces. It is a work in progress, most recently updated in 2012 to include the higgs boson.
Before we begin discussing the standard model we will provide some background information. to skip this select ‘The Standard Model’ below and begin reading from there.
The discovery of sub-atomic particles
Dirac’s Prediction of Antimatter
Fundamental Forces and Force Carrying Particles
Photons (y) – The Electromagnetic Force
Z and W Bosons – The Weak Force
The discovery of sub-atomic Particles
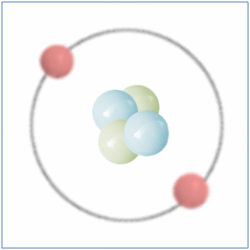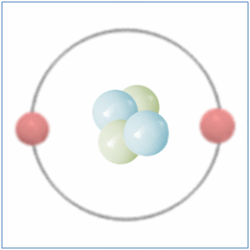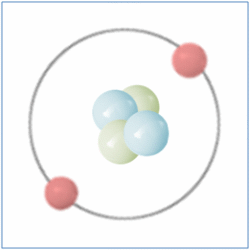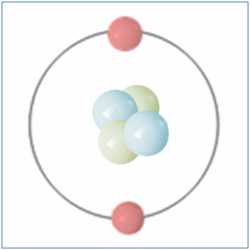 By the Late 19th and Early 20th Centaury scientist became aware that although all matter was made from atoms, atoms themselves were made up of sub atomic particles and the first of these to be discovered was the electron by J.J. Thomson in 1897. The electron is a negatively charged particle that orbits the nucleus of an atom.
By the Late 19th and Early 20th Centaury scientist became aware that although all matter was made from atoms, atoms themselves were made up of sub atomic particles and the first of these to be discovered was the electron by J.J. Thomson in 1897. The electron is a negatively charged particle that orbits the nucleus of an atom.
Later Ernest Rutherford performed a series of experiments between 1909 and 1911 which suggested that atoms also contained small, dense and positively charged nuclei. He named the new particle a Proton and it was believed for some time that these were the only particles that occupied the nucleus and gave the atom its mass.
In 1932 British Physicist James Chadwick proved the existence of the neutron. A neutron is a neutral particle that is similar in mass to the proton. By the mid 1930’s it had been widely accepted that matter was actually made up of three different sub-atomic particles that could be arranged in various ways to produce the elements of the periodic table. Further more the pattern found in the periodic table could now be explained. As well as the known three sub atomic particles scientist had also become aware of three fundamental forces that governed all matter. Gravity and its affects were widely known, Electromagnetic force was another and a third known as the strong force. This force was what held the nucleus together and had to be strong to be strong enough to overcome the electrical force that respells the positively charged protons in a nucleus. Three sub-atomic particles and three fundamental forces seemed elegant and sat very well with scientists of the day. But this model didn’t last for long.
Electron Shells
When creating his model of the Atom, Niels Bohr proposed that the electrons ‘orbited’ the Nucleus in a similar way to planets. This view of atoms has stuck and is the mode taught in schools but as understanding of physics involving the very small (quantum physics) increased it became apparent that this is not accurate.
Electrons actually sit in certain ‘energy levels’. Each ‘energy level’ a certain distance apart and able to hold varying amounts of electrons. The first energy level which is closest to the nucleus can hold two electrons while the next level holds eight – the electron capacity for the energy levels can be calculated with this formula – Electron Capacity = 2n2 (see the table below for energy levels)
Electrons are a type of fermion and therefore follow a rule that forces them to stay on the lowest energy level if possible. Electrons therefore will fill the first level and then continue to fill each level, one after another, until all there electrons have a place. In the case of Boron, which has 4 electrons it would have 2 electrons on the first energy level and another 2 on the next level. Carbon however has 6 electrons so it too fills the first energy level with 2 of its electrons and has four in the next.
Bohr realised that, from the point of view of the atoms, it would only be the outermost energy level that comes into contact with the outside world and it was the amount of electrons found on the outside energy level that had a direct effect on how the atom reacted with other atoms. This is why only certain atoms will combine and make molecules.
|
Energy Level (Principal Quantum Number) |
Shell Letter |
Electron Capacity |
|
1 |
K |
2 |
|
2 |
L |
8 |
|
3 |
M |
18 |
|
4 |
N |
32 |
|
5 |
O |
50 |
|
6 |
P |
72 |
The Photoelectric effect
Scientists had studied the properties of light for many centuries and it was work by Sir Isaac Newton that first made the suggestion that light was made of particles which he called ‘corpuscles’. Many years later a Dutch Physicist Christian Huygens suggested that light was not a stream of particles but a wave. This theory was ignored (largely because it was believed that this would make Newton wrong) but later experiments by Thomas Young showed without a doubt that light did indeed act like a wave.
Physicist had determined through experimentation that it was possible to produce electrons by shining a light on a piece of metal through a vacuum (a cathode ray tube) and it was theorised that the light was knocking electrons from the atoms of the metal. There was however a problem with this idea. When scientist intensified the light (usually by moving it closer) the theory was that the light should knock of electrons with more force and therefore they would be ejected with a higher velocity. This was not what was observed and could not be explained with classical physics. Even more curious was that the colour of the light had more effect on the velocity of the ejected electrons then its intensity.
In 1905 an unknown physicist by the name of Albert Einstein wrote several papers (all of which changed the views of physics) one of which was concerning the problem with the ‘photoelectric effect’, as it was known. He suggested, based on a paper by Max Planck, that light is made of small energy packets which he called quanta (from which we get the name Quantum Physics) and it is these packets that give the electron energy. The amount of energy in each packet is the same based on its frequency and therefore the intensity of the light has no effect. Additionally different colours of light have different frequencies and by changing the colour of the light you are changing the amount of energy in these quanta (now known as photons). The genius of this paper took some time to be realised but eventually it made scientist accept that light behaved as both waves and particles.
Further study in quantum physics showed that the photons supplied energy to the electrons and this makes the electron jump an energy level. Eventually electrons return to their energy state and in doing so they release energy and it is this effect that explains the lines seen in spectroscopy (black lines or ‘adsorption lines’ are the absorption of the energy when the electron jumps an energy level while the lines seen in ‘emission lines’ are the release of energy as an electron returns to a lower energy level).
The photoelectric effect is how energy is produced from solar panels.
Dirac’s Prediction of Anti-matter
In the 1920’s Physicist Paul Dirac considered the electron energy levels from a mathematical point of view. Mathematically speaking minus energy levels should exist and if electrons followed the rule of fermions and occupied the lowest energy level then it should occupy those minus energy levels first (see electron shells above). Dirac being the genius that he was chose not to ignore but to explore the possibilities of this problem. The only possible reason Dirac could give for this was if those minus energy levels did indeed exist invisibly in what we call space and they were already filled by invisible electrons. As already discussed above it is possible to make electrons jump to a higher energy level by supplying them with energy. If these ‘invisible electrons’ did exist then supplying them with enough energy should make them jump from the minus energy level and appear as a normal electron in our reality. This however creates a hole or space in the minus energy level which would appear as a particle of the same mass but with the opposite charge (in the case of an electron this would be a positive charge). Both of these particles would be created simultaneously but if the electron and its opposite (the hole) were to meet then the electron would fill into the whole. As with any electron falling to a lower energy level it would release energy of some sort. This is quite an extreme leap from the initial reasoning of a minus energy level existing but this is what happens. Scientists use the collision of two particle in a particle accelerator to produce the energy required to bring these electrons and their anti proton equivalent, a positron, into existence and they do indeed annihilate each other if they meet producing gamma radiation as they do so. Through increasing the speed of these particles before they collide scientists are able to increase the energy produced and they can produce more than just electrons through this same process, many of these particles have never been seen before and hundreds of different particles have been created but many are unstable and decay very quickly.
The discovery of anti-matter
Physicist Carl Anderson was experimenting with cloud chambers which are devices that show the movement of particles, such as electron, through the trail they leave in the gas. The moving electron, with a negative charge, could have its path bent by the attraction of a positive magnetic field. Holding the positive magnet near the chamber caused the negative electron to be attracted to the positive magnet but Anderson observed particles that moved away from the positive magnet. This suggested that the particle, which was the same mass as an electron, had a positive charge which caused it to be repelled from the positive magnetic field. This was the first evidence of Dirac’s predicted positron.
Another area of science that found many more types of particles was the discovery of cosmic rays. Many scientists used balloons to experiment on this new phenomenon and in 1937 a heavy electron was discovered. It had the mass of more than 200 electrons but was also negatively charged and is now known as a ‘muon’. In 1947 Cecil Powell discovered ‘Pions’.
Particle Accelerators
In April 1932 British Physicist John Cockroft and Irish Physicist, Ernest Walton, successfully built the world’s first particle Accelerator. A few weeks after Chadwicks discovery of the Neutron Cockcroft and Walton built their particle accelerator, also in Cambridge. They created a voltage multiplier (loosely similar to a transformer) to bombard Lithium with protons, neutrons and electrons which split the Lithium atom into other elements including helium. This is considered to be the first instance of synthetically splitting the atom.
These Particle Accelerators would accelerate charged particles and make them collide at colossal speeds and the most famous of these is the Large Hadron Collider (LHC) which accelerates particle around a 27km ringed chamber making them reach just 3 meters per second slower than the speed of light. The LHC is the largest of these particle accelerators but earlier models had given scientist many new particles to consider. They were quite often referred to as a particle zoo because of their number and variety. It was not as simple as finding these particles, scientist wanted to find a way to understand and categorize them.
When two baryons such as protons collide with enough force a new particle such as a pion was produced. This was because the kinetic energy of the first particle before it hit another was greater than the kinetic energy of the struck particle. This energy ‘loss’ was returned as matter (keeping with Einstein’s E=mc2) and this new matter would sometimes be a new particle. In fact the more force given to the first particle would create a larger ‘loss’ of energy and create more exotic particles.
Hadrons
The first thing they noticed about these new particles was that there were two distinct types Hadrons and leptons.
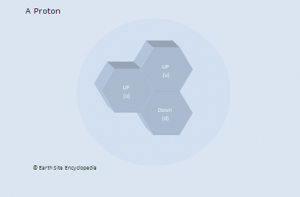
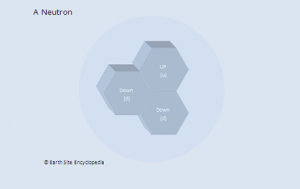
Hadrons are sub-atomic particles that are affected by the ‘strong force’. This includes particles such as protons (p+), neutrons (n0) or pions (p+, p0, p–). The only stable baryon is the proton. Even neutrons that are not bound within a molecule will decay (free neutrons have a half life of 15 minutes). Protons are the least massive of all baryons and this means it has the lowest rest mass/energy making it the most stable of all baryons.
Hadrons are either Baryons or Mesons.
Baryons
Baryons are a type of particle, as defined by the standard model of particle physics – also known as quantum field theory, which includes protons and neutrons. They are part of a larger group of particles known as Hadrons, all of which are affected by the strong nuclear force which binds them together. As its name suggests the strong nuclear force is the strongest of the four fundamental forces but only works very close range, for example, protons and neutrons need to be ‘touching’ for it to have any effect.
In collision reactions with Baryons it was found that the electrical charge is conserved and also the amount of baryons before the collision is the same after. For example in a collision with two protons colliding (as below) an extra meson may be created but no extra protons
p + p à p + p + p0
In some cases in a collision between two baryons can cause an additional baryon but it will always be accompanied by its anti-baryon which annihilates the additional copy (as below)
p + p à p + p + p + p–
So in Baryon collision reactions the electrical charge is conserved and the number of baryons (or Baryon Number) is also conserved.
Examples of Baryons
|
Name |
Symbol |
|
Proton |
p+ |
|
Neutron |
n0 |
|
Delta-plus |
D+ |
|
Delta-zero |
D0 |
|
Delta-minus |
D– |
|
Sigma-plus |
S+ |
|
Sigma-zero |
S0 |
|
Sigma-minus |
S– |
|
Lambda |
L |
Mesons
In The Standard model of Particle Physics mesons are a one of two types of particles known as Hadrons, the other being baryons. Baryons are the more common type of Hadron and include protons and neutrons while mesons include particles such as pions. Hadrons are made of three quarks combined together while mesons are made of a single quark combined with an anti-quark. Another distinction is that when baryons collide their number is conserved (see Baryons) while this is not the case with mesons.
Examples of Mesons
|
Name |
Symbol |
|
Phi |
F |
|
Pi-plus |
p+ |
|
Pi-zero |
p0 |
|
Pi-minus |
p– |
|
Kay or Kappa-plus |
K+ |
|
Kay or Kappa-zero |
K0 |
|
Kay or Kappa-minus |
K– |
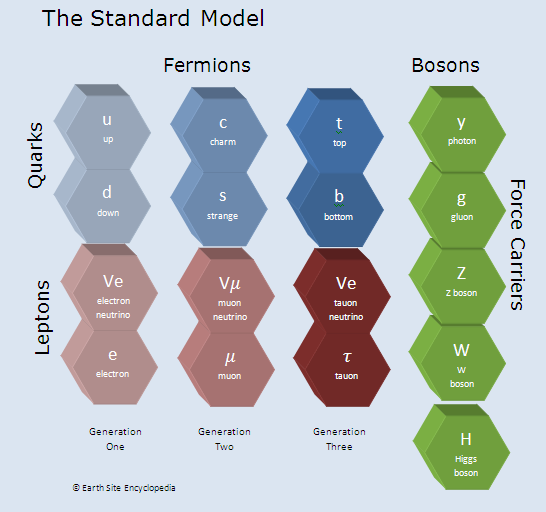
The Standard Model of Particle Physics
The Standard Model of Particle Physics or standard model is a very accurate model of quantum field theory. It has been tested for decades and made predictions, such as the existance of the higgs boson, which was discovered in 2012. The Standard Model was first developed in the 1970’s and has been able to explain almost all experimental results and accurate predict many phenomena.
Currently the Standard Model is our best description of the sub-atomic world but it is not complete. The standard model explains three of the fundamental forces (Electromagnetic, Strong Nuclear Force and Weak Nuclear Force) but doesn’t incorporate gravity. There are also some questions that are not explained by the standard model such as what happened to the antimatter after the big bang event and what ‘Dark Matter’ is.
The three sub-atomic particles that make up an atom comprising of Protons and neutrons at the centre with electrons orbiting around the outside. This is a well known and adequate method to explain much of chemistry but the standard model of quantum physics goes deeper to categorize what these particles are, the forces that act on particles and what they themselves are made of. According to the Standard Model all matter is made up of 12 fundamental particles and governed by four fundamental forces. For example Protons and Neutrons are a type of particle known as a Baryon and these are made up of three smaller particles called quarks.
Fermions
In The Standard model of Particle Physics particles can be placed in one of two groups, one is Fermions (named after Enrico Fermi who first proposed them) and the other is Bosons (named after Satyendra Bose who first proposed Bosons).
All particles that make up matter or ‘material’ particles are fermions while other ‘ghost’ particles, such as photons, are Bosons.
Fermions include Quarks (which make up and include protons and neutrons) and Leptons (which includes electrons). Fermions have half integer spin and it is because of this that different atoms react differently.
As the electrons are on the outer part of the atom it is the electrons that interact with the electrons of other atoms and allow them to combine to form molecules. The electrons fill the inner most ‘shell’, dependent on the space available, and then begin a new shell. Molecules are formed when the electrons on the outer shells of two or more atoms can combine and fill all the required spaces (see electron shells). Without this interaction atoms would not form molecules and the universe would be full hydrogen and helium atoms but not much else. It is because electrons (and all fermions) have a half integer spin that the electrons form these series of shells rather than all congregating on a single ‘orbit’ around the nucleus.
Quarks
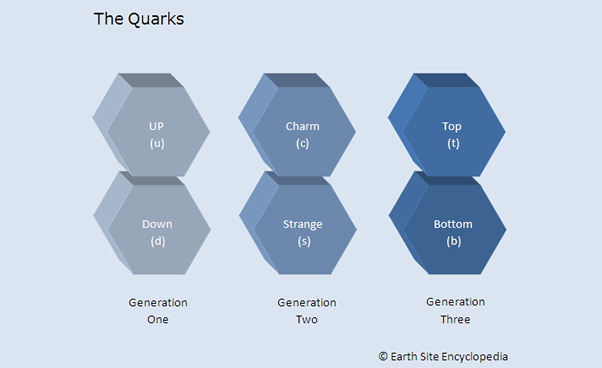
In 1964 American physicist Murray Gell-Man suggested that the observations from hadron reactions was because hadrons were not fundamental particles but were themselves made up of smaller particles which he named quarks.
These particles are affected by the strong force and include neutrons, protons and pions.
There are six deferent types of quarks and these are known as “flavours” (because they were originally named after flavours of ice-cream). The flavours are Up (u), Down (d), Charm (c), Strange (s), Top (t) and Bottom (b). The quarks flavours are paired into “generations” as shown in the diagram below.
Generation One
Up and down quarks are in the first generation and are the lightest of the quarks. These quarks make up all stable matter in the universe such as protons and neutrons.
Generation Two
Particles made from these quarks only last for a short time and are made up of heavier elements not normally found in nature. Once created they will decay quickly into first generation particles.
Generation Three
Particles made of top and bottom quarks are the heaviest of all and will decay even quicker, first into second generation and then into the stable first generation particles. Top Quarks were introduced into the standard model of particle physics in 1995.
To make baryons three quarks from a single generation are combined, but to make mesons only two quarks from different flavours or even quarks and anti-quarks are used.
Here are some more examples of how quarks combine to make particles.
Example of baryons
|
Baryon |
Symbol |
Quarks |
|
Proton |
p+ |
u, u, d (up, up ,down) |
|
Neutron |
n0 |
u, d, d (up, down, down) |
|
Delta-plus |
D+ |
u, u, d (up, up ,down) |
|
Delta-zero |
D0 |
u, d, d (up, down, down) |
|
Delta-minus |
D– |
d, d, d (down, down, down) |
|
Sigma-plus |
S+ |
u, u, s (up, up, strange) |
|
Sigma-zero |
S0 |
u, d, s (up, down, strange) |
|
Sigma-minus |
S– |
d, d, s (down, down, strange) |
|
Lambda |
L |
u, d, s, (up, down strange) |
Examples of mesons
|
Meson |
Symbol |
Quarks |
|
Phi |
F |
s, s¬ (strange, anti-strange) |
|
Pi-plus |
p+ |
u, d¬ (up, anti-down) |
|
Pi-zero |
p0 |
u, u¬ or d, d¬ (up, anti-up or down, anti-down) |
|
Pi-minus |
p– |
d, u¬ (down, anti-up) |
|
Kay or Kappa-plus |
K+ |
u, s¬ (up, anti-strange) |
|
Kay or Kappa-zero |
K0 |
d, s¬ (down, anti-strange) |
|
Kay or Kappa-minus |
K– |
s, u¬ (strange, anti-up) |
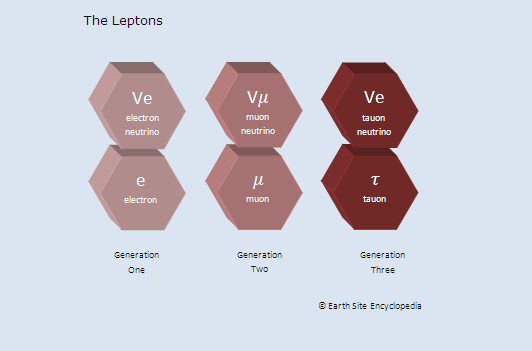
Leptons
Leptons are another type of matter particle with the most commonly known being the electron. Leptons are not affected by the strong force but are affected by the electromagnetic and weak forces. Examples of these include electrons, neutrinos and muons.
Leptons are setup similarly to quarks in that they also contain generations.
Generation 1
This pair consists of the electron and electron neutrino and these are the lightest of the generations.
Generation 2
The muons are about 200 times more massive than the electrons
Generation 3
The tauon particles are 3,500 times more massive than the electron.
Also the electron, muon and tauon have a charge of -1 while their neutrino pair have no charge at all. The tau neutrino was introduced into the standard model of particle physics in 2000.
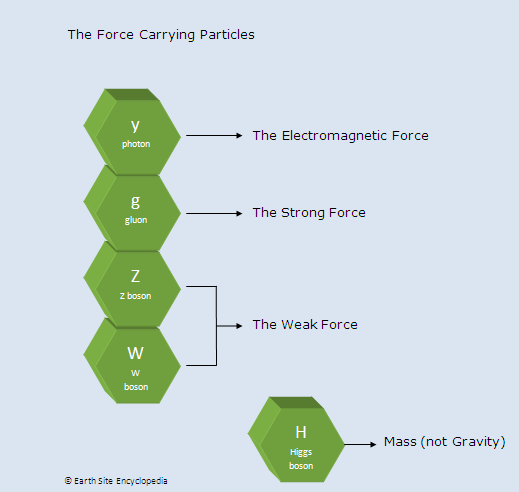
Fundamental Forces and Force Carrying Particles
Bosons
In the standard model of particle physics particles can be placed in one of two groups, one is Fermions (named after Enrico Fermi who first proposed them) and the other is Bosons (named after Satyendra Bose who first proposed Bosons).
All particles that make up matter or ‘material’ particles are fermions while other ‘ghost’ particles, such as photons, are Bosons.
Boson particles have either no spin or integer spin (as opposed to half-integer spin particles which are fermions). These particles follow a set of rules which were were worked out by Satyendra Bose and Albert Einstein and these rules are known as Bose-Einstein Statistics. Bose began the process by assigning statistical laws to massless particles and this was built upon by Einstein.
Bosons are massless particles but can interact with other particles as if they had mass. When a photon for example hits an electron it can pass energy to that electron which is expressed as momentum. This was shown in experiments with X-rays performed by Arthur Compton in the 1920’s. He showed that when X-rays (photons) collided with electrons they transferred momentum in a similar way to one snooker ball striking another. When this happens in snooker the ball that strikes another looses the transferred energy and its momentum decreases. In Compton’s experiment the photon passed on momentum like a particle but lost energy through changing its frequency like a wave. This experiment showed perfectly that light is a duality of both waves and particles.
Photons (y) – The Electromagnetic Force
This particle is responsible for the electromagnetism which affects charged particles such as protons and electrons. It is the attractive and repulsive force as found in magnetism and electrical charges. Electromagnetic force also determines the atomic structure which allows certain atoms to bind together and create molecules.
In 1905 Albert Einstein suggested that light is made of small energy packets which he called quanta (from which we get the name Quantum Physics). The amount of energy in each packet is the same based on its frequency (the colour of the light). These packets of quanta are now known as Photons.
Further study in quantum physics showed that the photons supplied energy to the electrons and this makes the electron jump an energy level. Eventually electrons return to their energy state and in doing so they release energy and it is this effect that explains the lines seen in spectroscopy (black lines or ‘adsorption lines’ are the absorption of the energy when the electron jumps an energy level while the lines seen in ‘emission lines’ are the release of energy as an electron returns to a lower energy level).
Colours are produced when certain frequencies or colours of light are absorbed by the electrons of an object. The colours that are not absorbed are reflected and picked up by the eye.
Gluons (g) – The Strong Force
Gluons are mass less particles responsible for the strong force which binds quarks together to form neutrons and protons. It also binds neutrons and protons together in the nucleus of atoms. The strong force is the strongest of all the fundamental forces and is 102 times stronger than the electromagnetic force. But the strong force has a very limited range extending only as far as the nucleus on an atom which is 10-15m.
Z and W Bosons – The Weak Force
The weak force is about 1010 times weaker than the electromagnetic force and has a shorter range than the strong force. Sometimes known as the weak nuclear force because it is affects are most prominent in nuclear reactions. A neutron in an unstable isotope is made more stable by the weak force which changes one of the quarks (neutron = d, d, u and proton = u, u, d) making the neutron into a stable proton (as previously stated a proton has the least mass and is the most stable of all baryons. Free neutrons have a half life of 15 minutes but are made stable when part of stable nuclei). As the mass of a neutron is greater than a proton the ‘lost’ mass produces a high energy, negatively charged, electron in a process called beta decay.
Gravity and the Higgs Boson
The force of gravity is probably the most well known of the fundamental forces but it has not yet been explained in the standard model. It is the weakest of all the forces at about 1040 times weaker than the strong force, but it has got an infinite range.
The Higgs Boson was the theoretical particle (proposed in 1964) responsible for the mass of matter and was discovered on the 4th of July 2012 at the Large Hadron Collider.
Newton stated that gravity is equal to the mass of the two bodies divided by the distance between their centres. His theory proved to be incredibly accurate and it was using this calculation, and other Newtonian Mechanics, that mankind was able to put a man on the moon some 240 years after his death. Despite his accuracy Einstein believed this was not the full picture. In 1965 Einstein published his paper on general relativity which claimed that the mass of objects warped the fabric of space-time and therefore the geometry that objects within the vicinity had to travel. If Newton’s theory of gravity was definitive than gravity would not affect light which has no mass but if Einstein was correct light would be bent when it passed through the warped space around a large object.
On the 29th of May 1919, during a total solar eclipse, Sir Arthur Eddington measured the position of stars with a line of sight ran very close to the sun. The sun was the only body with enough mass to show detectable warping of light but it would normally prove impossible due to the intensity of the sun’s light. The eclipse provided the perfect setting for the experiment as the sun’s light was blocked. By measuring stars which had a line of sight which ran close to the sun it should have been possible to detect warping which would have been visible by a shift in the stars normal apparent position. The experiment did show the warping effect consistent with Einstein’s prediction and the following morning Einstein was a worldwide celebrity.
It may be possible that superstring theory will be able to unify gravity with the other fundamental forces, completing the standard model and giving us a consistent quantum theory of gravity.
For more information on the quantum world I recommend reading“In Search of Schrodinger’s Cat” by John Gribbin.