Deoxyribonucleic acid or DNA is found in every cell of every living organism on the planet. It is the code which contains the genetic makeup of the organism.
The Structure of DNA
Polynucleotides
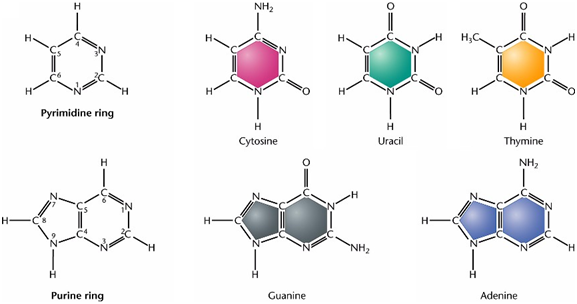
Dideoxynucleic acid molecule consisting of three components: a nitrogenous base, either a purine or pyrimidine; a pentose sugar, either ribose or deoxyribose and a phosphate group.
A nitrogenous base + a sugar= nucleoside; a nucleoside + a phosphate= nucleotide.
Purine: nine member double ring: Adenine and Guanine (A and G)
Pyrimidine: six member single ring: Cytosine and Thymine or Uracil in Rna (C, T and U)
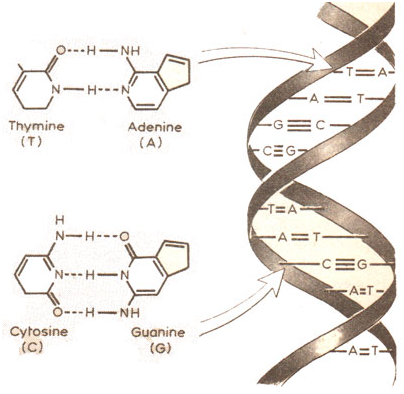
A purine bases will always bind to its corresponding pyrimidine base: A-T/U and C-G.
Structure of DNA
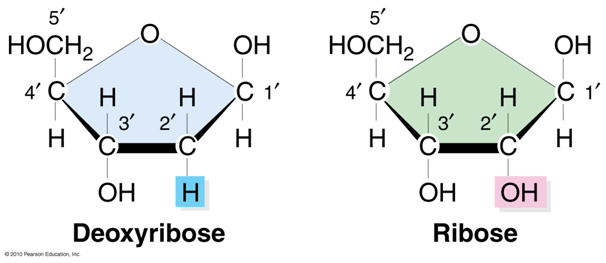
Each carbon atom of the pentose sugars is labelled with a prime sign eg. C-1’. The C-1’ atom of the pentose sugar is linked to the nitrogenous base and
C-3’ and 5’ atoms are termini of a nucleic acid molecule. At the C-2’ position, deoxyribose has a hydrogen atom whereas ribose has a hydroxyl group. The presence of the OH group distinguishes RNA from DNA.
http://www.mun.ca/biology/scarr/iGen3_02-07_Figure-L.jpg
Two nucleotides are joined together by a phosphate group linked to two sugars. The phosphoric acid is joined to the two OH groups on the sugars by an ester linkage on both sides, creating a phosphodiester bond. At one end of the chain there is a free 3’- hydroxyl (OH) group and at the other end, a free 5’- phosphate group.
Two nucleotides joined together are called a dinucleotide, three is a trinucleotide and so on. Short chains of around 20 nucleotides are called oglionucleotides and longer chains are known as polynucleotides.
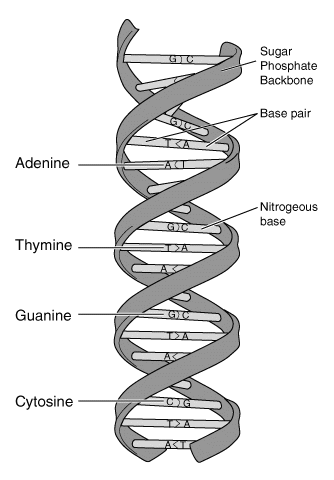
DNA consists of two long polynucleotide chains that are coiled round a central axis to form a right- handed double helix. The chains run anti-parallel e.g. C-5’ to C-3’ orientations run in opposite directions. Two types of DNA molecules: right handed and the left handed mirror image referred to as Z-DNA. This contains only Cytosine and Guanine bases.
The helical structure is maintained by hydrogen bonding between the bases. Hydrogen bonds are very weak electrostatic attractions between a covalently bonded hydrogen atom and an atom with an unshared electron pair such as a covalently bonded oxygen or nitrogen atom e.g. O2– ….H+