It has been believed for a thousand years that atoms were the smallest component of matter, but in the late 19th century that the first evidence that atoms were made up of smaller sub-atomic particles came to light.
About Atoms
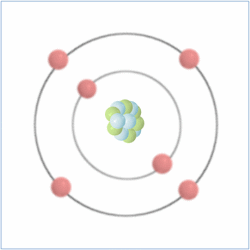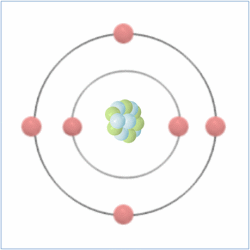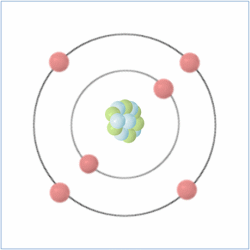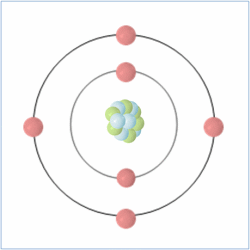
It has been known for a thousand years that all matter is made up from small particles known as atoms but for much of this time it was believed that atoms were the smallest component of matter. It was not until the late 19th and early 20th century that the first evidence that atoms were made up of smaller sub-atomic particles came to light.
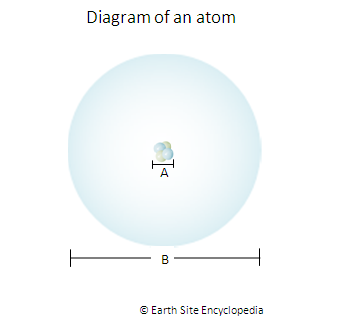
A = The Nucleus of the atom contains the protons and neutrons. Despite accounting for the majority of an atom’s mass, the nucleus procures a minute proportion of the total space. The Diameter of the nucleus is approximately 4 femtometres or 4 x 10-15 meters. It is very difficult to measure the diameter of the nucleus because like our atmosphere it has no define edge but gradually fades away.
B = this is mainly just empty space where the electrons orbit the nucleus. Its diameter (and that of the whole atom) is 0.1 nanometres or 0.1 x 10-9 meters.

Here is the chemical symbol for helium as you might see it on a periodic table. The number at the top is known as the atomic number and is actually the number of protons that are found in the atom while the atomic mass is the total number of protons and neutrons (collectively called nucleons) combined. Therefore you can calculate the number of neutrons by taking the atomic number from the atomic mass.
Electrons
Electrons are found orbiting the atomic nucleus (made up of protons and neutrons) and are said to be in shells. The negatively charged electrons are attracted to the positively charged protons in the nucleus through electromagnetic force which hold them in orbit. The extreme pressure of the sun creates enough heat energy to break the electromagnetic force of the atoms and allow the electrons to float freely. When matter acts like this its ‘state of matter’ is known as plasma (the four ‘states of matter’ are solid, liquid, gas and plasma).
Discovery of Electrons

It had been previously suggested that atoms may actually be made up of smaller particles but no one predicted how minute they were until British physicist J.J. Thomas. J.J. Thomas began experiments with cathode rays and proved on the 30th of April 1897 the existence of the electron.
Properties of Electrons
Electrons are negatively charged sub-atomic particles which are normally found in equal quantities to the protons (positively charged sub-atomic particles) in elements which balance their charge making them “neutral atoms”. Sometimes atoms do have more or less electrons than protons which make them positively or negatively charged atoms known as ‘ions’.
They are the lightest particle found in the atom and have so little mass (approximately 1,000 times less massive than a proton or neutron at 9.109 × 10−31 kg) they are generally considered to have no mass (when considering the mass of the entire atom). Therefore the mass of an atom is based on the amount of nucleons (the collective name for neutrons and protons) and doesn’t take into account the amount of electrons.
Within Particle Physics electrons are types of Leptons and are therefore affected by electromagnetic, gravitational and weak forces (three of the four fundamental forces). These making them fundamentally different from protons and neutrons which are made up of quarks and are governed by the strong force.
Protons
Protons are found within the nucleus of every element on the periodic table and determine the properties of that atom. By this we mean that increasing the protons changes the element (increasing the neutrons changes the atom into another isotope of that element but doesn’t change into another element). A proton is signified by using the lowercase letter p or by using the letter p followed by a superscripted plus sign ( p+ ) to show that it is positively charged. All protons are positively charged but have a anti-particle equivalent known as an antiproton which has a negative charge. Protons are the most stable baryon (a baryon is the name for a particle that is made from quarks). If neutrons are free flowing (such as in plasma where they may be found outside the safety of a nucleus) they will decay into protons. Free neutrons have a half-life of about 15 minutes and will decay into the more stable and slightly less massive Proton through beta decay (producing an additional electron).
Discovery of Protons

Soon after, in 1909, the great New Zealand born British physicist Ernest Rutherford began his most famous experiment. He and two students, Hans Geiger (Geiger counter) and Ernest Marsden, fired alpha particles at some gold foil and detected that in some instances the alpha particles were deflected. According to the theory of atoms at the time the alpha particles should have passed through the foil without obstruction. On the 7th of March 1911 Rutherford presented his findings to the Manchester Literary and Philosophical Society where he first publically described the Rutherford model of an atom which contained a relatively large, heavy and positively charged nucleus which was surrounded by Thomas’s electrons orbiting around with a large amount of space between. His paper was later published in May that year (Philosophical magazine, series 6, vol. 21, page 669-688) and it changed the understanding of the atom dramatically. Furthermore in 1919 Rutherford fired alpha particles at nitrogen atoms and became the first person to transmute an element when he changed the nitrogen into oxygen. He noticed that another particle was released also. This particle was the previously unknown proton but Rutherford had detected it before as the hydrogen atom. This led him to deduce that the component of hydrogen was also present in nitrogen and probably in all elements.
Properties of a Proton
The number of protons in an atom changes its qualities making it become a different element.
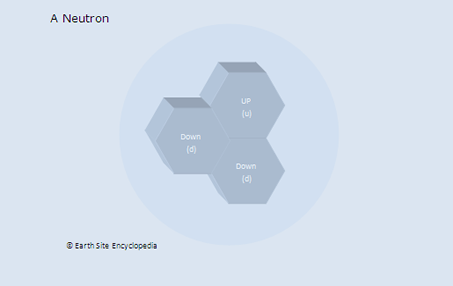
Neutrons
Neutrons are present in all elements but basic hydrogen which contains just one proton and one electron. Both deuterium and tritium, which are isotopes of hydrogen, contain neutrons. Increasing the number of neutrons without increasing the number of protons doesn’t change one element into another but it does change an element into one of its isotopes. Neutrons are less stable than protons when not contained in the nucleus (such as when the state of the matter is plasma). This is because the mass of a free flowing or isolated neutron is slightly greater than a proton. Free neutrons have a half-life of about 15 minutes and will decay into the more stable and slightly less massive Proton through beta decay (producing an additional electron).
Discovery of Neutrons
 In 1932 British Physicist James Chadwick (who studied first under Rutherford and then under Hans Geiger) discovered the Neutron. Rutherford had predicted its existence in 1922 but it would be his former student that would prove it. Physicists knew, for example, that the nucleus of nitrogen had an atomic mass of 14 but a charge of +7. To explain this they proposed that nitrogen atoms had 14 protons and 7 electrons in the nucleus (as well as the 7 electrons that orbited) and these 7 negatively charged electrons in the nucleus would neutralise 7 of the protons which explained its +7 charge. Chadwick argued that the nucleus actually contained particles that weighed the same as protons but had no charge which would give the same result. He performed several experiments at the University of Cambridge, England which proved his theory and thus the Neutron was discovered.
In 1932 British Physicist James Chadwick (who studied first under Rutherford and then under Hans Geiger) discovered the Neutron. Rutherford had predicted its existence in 1922 but it would be his former student that would prove it. Physicists knew, for example, that the nucleus of nitrogen had an atomic mass of 14 but a charge of +7. To explain this they proposed that nitrogen atoms had 14 protons and 7 electrons in the nucleus (as well as the 7 electrons that orbited) and these 7 negatively charged electrons in the nucleus would neutralise 7 of the protons which explained its +7 charge. Chadwick argued that the nucleus actually contained particles that weighed the same as protons but had no charge which would give the same result. He performed several experiments at the University of Cambridge, England which proved his theory and thus the Neutron was discovered.
Properties of Neutrons
Changing the number of Neutrons in an atom changes the element into a different isotope of that element.