
Beryllium (named after the mineral, beryl, that it was originally isolated from)

Classification: Alkali earth metal
Atomic Mass: 9.012182 (3) g/mol
Density: 1.85g/cm3
Colour: grey
Boiling Point: 2742K (2469°C)
Melting Point: 1560K (1287°C)
Discovery of Beryllium
In 1798 French chemist René Haüy noticed similarities in the crystal structures and properties of beryl and emerald despite their differences in colour, a fact that had been noted by the ancient Egyptians. But René Haüy deducted that this was due to them both containing the same as yet unknown element and approached a specialist in chemical analysis Nicolas Louis Vauquelin. At the time it was common practise to taste substances as part of the analysis and Vauquelin tasted the substance which was sweet, and unknown to him toxic, so Vauquelin named the substance ‘glyceynum’ from the Greek word ‘glykis’ meaning sweet.
Beryllium was isolated separately by French chemist Antoine Alexandre Bussy and German physician and chemist Friederich WÖhler in 1828. They both reacted beryllium chloride and potassium in a platinum crucible and yielded beryllium and potassium chloride. Friederich Wöhler renamed the element ‘beryllos’ from the mineral beryl however Bussy preferred the name ‘glucinium’ and it wasn’t until 1957 that it was officially called beryllium.
Sources
Beryllium occurs naturally and can be found in bertrandite and Beryl ore. The United States, China and Kazakhstan are the only countries that commercially process the ore and produce it from exposing the ore to sulphuric acid to produce a sulphate and refined through a chemical process which produce Beryllium Hydroxide.
Uses
Beryllium is a strong lightweight metal which is non magnetic, can absorb immense amounts of heat and resists structural change in extremely cold temperatures.

Beryllium is used by NASA to make mirrors for the Webb Telescope. The telescope is to be sent into outer space requiring a substance that will keep its shape in the cold temperatures of space about -228°c or -379°f. The mirrors in the image on the left have just been tested at NASA’s X-ray & Cryogenic Facility to ensure they maintain their shape at -248°c or -415°f.
Beryllium can withstand very high temperatures with a melting point of 1287 °C it is often used in rockets, missiles and satellites.
The materials high heat capacity also makes it an ideal for heat sinks in electronic devices.
Additionally it is also transparent to X-rays and so is used as windows for x-ray equipment.
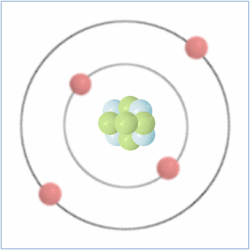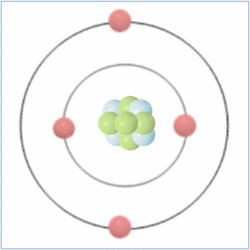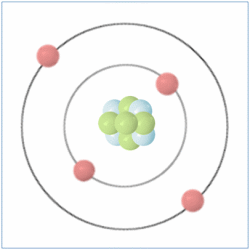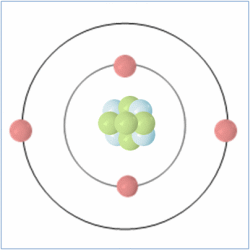
Shell Structure
Protons = 3
Neutrons =4
Electrons=3
|
s |
p |
d |
f |
|
|
1 |
2 |
|||
|
2 |
2 |
|||
|
3 |
||||
|
4 |
||||
|
5 |
||||
|
6 |
||||
|
7 |
Absorption Lines
Emission Lines