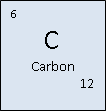
Carbon (from the latin word ‘carbo’ meaning coal)

Classification: Non-metal (solid at 298 K)
Atomic Mass: 12.0107 g/mol
Density (graphite): 2.267g/cm3
Density (Diamond): 3.513g/cm3
Colour: Diamond has no colour / graphite is black
Boiling Point: 4300K (4027°C)
Melting Point: 3800K (3527°C)
Discovery
The production of carbon in the form of charcoal has been known since records began.
Sources
Charcoal was produced by burning organic material in an atmosphere with reduced oxygen. Although it is possible to make superb diamonds artificially the most precious kind are produced naturally under extreme pressure over a very long period of time.
Uses
The ratio of carbon on earth is thought to be 99% carbon-12, just under 1% Carbon-13 and 0.0000000001% of carbon-14 with the same ratios found in all living things. Carbon-14 is an unstable isotope of carbon which can be used in the process called carbon dating. As all living things intake carbon in the above ratios, when an organism dies the Carbon 14 begins to decay. This means that over time the carbon 14 ratio decreases over time and this decrease can be measured. As scientists know the decay rate of Carbon 14 they can measure the approximate time since the organism died.
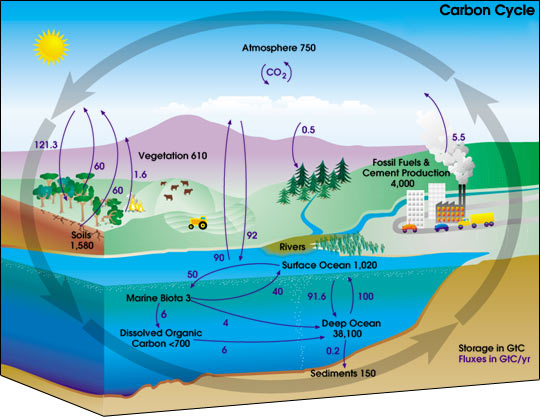
Carbon is one of the fundamental building blocks of life and most carbon would have been through a process we call the Carbon Cycle. This is were carbon from the atmosphere passes into plants through photosynthesis, then into animals through the food chain and into the ground through decomposition when the life forms die.
Carbon fibres are a very strong and lightweight material used in the aerospace industry. It is created by heating certain materials such as polyacrylonitrile to high temperatures and by doing so its structure changers and impurities such as hydrogen and nitrogen are boiled off creating an increasingly pure and wider ribbons as the minute chains combine together.
Diamonds are not formed by coal as commonly thought and in fact most diamonds were formed before plant life and therefore any possibility of coal existed.
Diamonds are formed from the same element (carbon) but under immense pressure and heat energy. Normally only found naturally in the depth of the earth or in outer space. For more information see diamond formation.
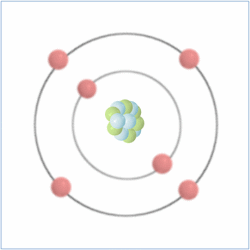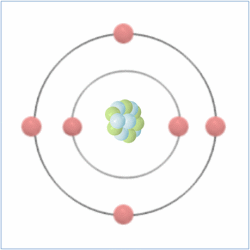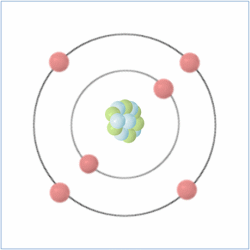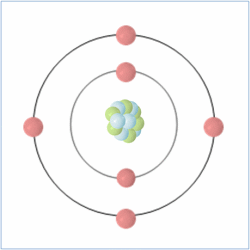
Shell Structure
Protons = 6
Neutrons = 6
Electrons= 6
|
|
s |
p |
d |
f |
|
1 |
2 |
|
|
|
|
2 |
2 |
2 |
|
|
|
3 |
|
|
|
|
|
4 |
|
|
|
|
|
5 |
|
|
|
|
|
6 |
|
|
|
|
|
7 |
|
|
|
|
Absorption Lines

Emission Lines