🧪 Introduction to Chemistry
Unlocking the Secrets of Matter and Change
Chemistry is the science of matter—what it’s made of, how it behaves, and how it changes. It explores everything from the tiniest atoms and molecules to the vast chemical reactions that fuel stars, power engines, and sustain life itself. Often called the “central science,” chemistry connects physics with biology, medicine, geology, environmental science, and even engineering.
At its core, chemistry seeks to answer questions like:
-
What is this substance made of?
-
How does it interact with other substances?
-
Why do some materials burn, rust, or dissolve?
-
How can we create new materials, medicines, or fuels?
From the food we eat to the air we breathe, from cleaning products to smartphones, chemistry is everywhere. It helps explain natural phenomena like fire, digestion, and photosynthesis, while also driving innovations in technology, health, and sustainability.
By studying chemistry, we gain a deeper understanding of the world at a molecular level—and the tools to change it for the better.
The Power of Hydrogen: Exploring the Potential of H2 as a Clean Energy Source
Clean energy has become a pressing issue in today’s world as we face the challenges of climate change and the depletion of fossil fuels. The need for sustainable and renewable sources of energy has never been more urgent. One potential solution to this problem is hydrogen, a versatile and abundant element that has the potential to revolutionize the way we produce and consume energy. Hydrogen is often referred to as the “fuel of the future” due to its abundance and clean burning properties. It is the most abundant element in the universe and can be found in water, organic matter, and fossil fuels. When burned, hydrogen produces only water vapor, making it a clean and environmentally friendly source of energy. Summary Clean energy is important for a sustainable future Hydrogen is a versatile and abundant element with unique properties Hydrogen can be produced through various methods, including electrolysis and steam methane reforming Fuel cells convert hydrogen into electricity with high efficiency and low emissions Hydrogen storage remains a challenge, but solutions such as compressed gas and liquid hydrogen are being developed The Science Behind Hydrogen: Properties and Characteristics Hydrogen is the lightest and simplest element in the periodic table, with an atomic number of 1. It consists of a single proton and a single electron, giving it a neutral charge. At room temperature, hydrogen exists as a gas and is colorless, odorless, and tasteless. One of the most unique properties of hydrogen is its high energy content. It has three times more energy per unit mass than gasoline, making it an efficient fuel source. Additionally, hydrogen has a high...
Unveiling the Secrets of the Universe with Spectroscopy: A British Perspective
Spectroscopy is a powerful scientific tool that has revolutionized our understanding of the universe. It involves the study of the interaction between matter and electromagnetic radiation, allowing scientists to analyze the composition, structure, and properties of various substances. From its early beginnings with pioneers like Isaac Newton and William Hyde Wollaston, spectroscopy has come a long way and continues to play a crucial role in scientific research. This blog post will explore the history, current applications, and future trends of spectroscopy in Britain. Summary Spectroscopy has a rich history in Britain, dating back to the work of Isaac Newton in the 17th century. Spectroscopy is revolutionizing our understanding of the universe, allowing us to study everything from distant galaxies to the composition of exoplanet atmospheres. British universities have played a key role in advancing spectroscopic research, with institutions like Cambridge and Oxford leading the way. Spectroscopy is being used in the search for extraterrestrial life, as scientists analyze the spectra of exoplanet atmospheres for signs of biological activity. Conducting spectroscopic studies in space presents unique challenges, including the need for specialized equipment and the effects of cosmic radiation. The History of Spectroscopy in Britain: From Newton to the Present Day The roots of spectroscopy can be traced back to the 17th century when Sir Isaac Newton conducted experiments with prisms and discovered that white light could be separated into its component colors. This laid the foundation for the study of light and its interaction with matter. In the early 19th century, British scientist William Hyde Wollaston developed the first spectrometer, a device used to measure the intensity of different...
Exploring the Wonders of Chemistry: Strategies for Effective Teaching and Learning
Chemistry education plays a crucial role in our society, as it provides us with a deep understanding of the world around us. From the air we breathe to the food we eat, chemistry is involved in every aspect of our daily lives. It is the study of matter, its properties, and the changes it undergoes. Chemistry education equips individuals with the knowledge and skills to make informed decisions and contribute to various industries such as healthcare, energy, and environmental sciences. In this article, we will explore the importance of chemistry education, from understanding the building blocks of matter to exploring the principles of green chemistry. Summary Chemistry education is important because it helps us understand the world around us and make informed decisions. The basics of chemistry involve understanding the building blocks of matter, such as atoms and molecules. Chemical bonding is fundamental to understanding how molecules are formed and how they interact with each other. The periodic table is a comprehensive guide to the elements and their properties. Balancing chemical equations and predicting products are important skills for understanding chemical reactions. The Importance of Chemistry Education: Why It Matters Chemistry is relevant in everyday life, as it helps us understand the world around us and make informed decisions. For example, understanding the properties of different materials allows us to choose the right materials for construction or manufacturing processes. Chemistry also plays a crucial role in healthcare, as it helps develop new drugs and treatments for various diseases. Without a solid foundation in chemistry education, individuals may not fully comprehend the potential risks and benefits associated with different substances....
Exploring the Fascinating World of Computational Chemistry: A British Perspective
Computational chemistry is a branch of chemistry that uses computer simulations and mathematical models to study chemical systems. It involves the application of theoretical methods, such as quantum mechanics and molecular dynamics, to understand and predict the behavior of molecules and materials. Computational chemistry plays a crucial role in modern research, as it allows scientists to explore chemical phenomena that are difficult or impossible to study experimentally. The field of computational chemistry has a rich history, dating back to the 1950s. Early pioneers in the field, such as John Pople and Walter Kohn, laid the foundation for the development of computational methods that are still widely used today. Over the years, computational chemistry has evolved and expanded, with advancements in computer hardware and software enabling more complex simulations and calculations. Summary Computational chemistry is the use of computer simulations to study chemical systems and processes. The UK has a long history of contributions to computational chemistry, with notable advancements in the use of supercomputers. Supercomputers are essential for performing complex calculations in computational chemistry, allowing for the study of large molecules and systems. Computational chemistry has numerous applications in drug discovery and materials science, including the design of new drugs and materials with specific properties. Quantum mechanics and molecular dynamics are important tools in computational chemistry, allowing for the study of chemical reactions and the behaviour of molecules. The Evolution of Computational Chemistry in the UK The United Kingdom has been at the forefront of computational chemistry research since its inception. In the 1960s, the University of Cambridge became a hub for computational chemistry, with researchers like John Pople...
The Perils of Radioactivity: Navigating the Hazards of Nuclear Energy
Radioactivity and nuclear energy are topics that have gained significant attention in recent years. With the increasing demand for energy and the need to find sustainable and clean sources of power, understanding radioactivity and nuclear energy is crucial. This article aims to provide a comprehensive overview of the history of radioactivity, the dangers of radioactive exposure, the Fukushima disaster and its lessons, safety measures and regulations in nuclear power plants, challenges and solutions in radioactive waste management, the threat of nuclear proliferation, alternative energy sources, the role of science in understanding radioactivity, public perception of nuclear energy, and the future of nuclear energy. Summary Radioactivity was discovered in the late 19th century by Marie and Pierre Curie. Exposure to radioactive materials can cause cancer, genetic mutations, and other health problems. The Fukushima disaster highlighted the importance of safety measures and regulations in nuclear power plants. Radioactive waste management remains a major challenge, with no perfect solution yet. Nuclear weapons pose a significant threat to global security and stability. The History of Radioactivity: From Discovery to Nuclear Energy The discovery of radioactivity can be attributed to several scientists, including Henri Becquerel, Marie Curie, and Ernest Rutherford. In 1896, Becquerel accidentally discovered that uranium salts emitted rays that could penetrate opaque materials. This discovery led to further research by Marie Curie, who coined the term “radioactivity” and identified two new elements, polonium and radium. Ernest Rutherford later conducted experiments that led to the discovery of alpha and beta particles. Early uses of radioactive materials included medical applications such as cancer treatment and diagnostic imaging. However, it was not until the 20th...
Exploring the Advancements in Electronic and Optical Materials: A British Perspective
Electronic and optical materials play a crucial role in modern technology, enabling the development of devices such as smartphones, computers, solar cells, and medical imaging equipment. These materials have revolutionized various industries and have become an integral part of our daily lives. British research has played a significant role in advancing this field, with key contributions from institutions and researchers in the UK. This article will explore the latest developments in electronic and optical materials, highlighting the role of British research in shaping the future of this field. Summary British research has played a crucial role in advancing electronic and optical materials. Graphene and other 2D materials are at the forefront of research and development in this field. Advanced solar cells have the potential to revolutionize the energy industry. OLEDs and quantum dots are among the most promising technologies for the future of display technology. Electronic and optical materials have innovative applications in healthcare, such as biosensors and drug delivery systems. The Role of British Research in the Advancement of Electronic and Optical Materials British research has a rich history of contributions to the field of electronic and optical materials. In the 19th century, Michael Faraday’s work on electromagnetism laid the foundation for modern electronics. In the early 20th century, Sir J.J. Thomson’s discovery of the electron revolutionized our understanding of atomic structure and paved the way for the development of electronic devices. Key institutions in the UK have been at the forefront of research in electronic and optical materials. The University of Cambridge, for example, has been a hub for groundbreaking research in this field. Notable researchers such...
Exploring the Benefits of Green Chemistry in the UK: A Sustainable Solution for a Greener Future
Green Chemistry is a scientific approach that focuses on the design and development of chemical products and processes that are environmentally friendly, economically viable, and socially responsible. It aims to minimize the use and generation of hazardous substances, reduce waste, and promote sustainable practices. Green Chemistry is becoming increasingly important in today’s world as we face pressing environmental challenges such as climate change, pollution, and resource depletion. The principles of Green Chemistry include the use of renewable resources, the prevention of waste generation, the design of safer chemicals and processes, and the use of energy-efficient methods. By adopting these principles, Green Chemistry seeks to minimize the negative impact of chemical products and processes on human health and the environment. It also aims to promote the development of sustainable alternatives that can meet our societal needs without compromising future generations. Summary Green chemistry is a sustainable approach to chemical production that aims to reduce or eliminate the use of hazardous substances and waste. Sustainability is a key priority for the UK, with a focus on reducing carbon emissions, improving resource efficiency, and protecting the environment. Green chemistry can help to achieve sustainability goals by reducing the environmental impact of chemical production and promoting the use of renewable resources. Green chemistry offers several advantages over traditional chemistry, including lower costs, improved efficiency, and reduced environmental impact. Green chemistry has the potential to drive economic growth, improve public health, and foster innovation in the UK, but there are also challenges to be addressed, such as the need for investment in research and development. The Importance of Sustainability in the UK Sustainability is...
Unravelling the Mysteries of Statistical Mechanics: A British Perspective
Statistical mechanics is a branch of physics that aims to explain the behavior of macroscopic systems by studying the statistical properties of their microscopic constituents. It provides a framework for understanding the thermodynamic properties of matter and has applications in a wide range of fields, from condensed matter physics to biology. The development of statistical mechanics has been a collaborative effort by scientists from around the world, and British physicists have made significant contributions to this field. Summary British scientists have made significant contributions to the development of statistical mechanics. Statistical mechanics plays a crucial role in understanding complex systems. British researchers have provided unique perspectives on the statistical mechanics of thermodynamics, quantum systems, soft matter, and biological systems. The future of statistical mechanics presents both challenges and opportunities for British research. Overall, British research has been and continues to be important in the field of statistical mechanics. The Historical Development of Statistical Mechanics in Britain The history of statistical mechanics in Britain can be traced back to the 19th century, when scientists began to explore the relationship between the microscopic properties of matter and its macroscopic behavior. One of the key figures in this early period was James Clerk Maxwell, who formulated the kinetic theory of gases. Maxwell’s work laid the foundation for statistical mechanics by showing that the macroscopic properties of gases, such as pressure and temperature, could be explained in terms of the motion and interactions of their constituent particles. Another important figure in the development of statistical mechanics in Britain was Lord Kelvin (William Thomson). Kelvin made significant contributions to the understanding of thermodynamics and...
Unraveling the Mysteries of Chemical Structure and Bonding: A Comprehensive Guide
Chemical structure refers to the arrangement of atoms and the bonds between them in a molecule. Chemical bonding, on the other hand, is the process by which atoms are held together to form molecules or compounds. Understanding chemical structure and bonding is crucial in the field of chemistry as it allows scientists to predict and explain the behavior of substances. The importance of understanding chemical structure and bonding lies in its ability to provide insights into the properties and behavior of substances. By knowing the arrangement of atoms and the types of bonds present, scientists can determine the physical and chemical properties of a substance, such as its melting point, boiling point, reactivity, and solubility. This knowledge is essential in various fields, including pharmaceuticals, materials science, environmental science, and many others. Summary Chemical structure and bonding are fundamental concepts in chemistry. Atomic structure is the foundation for understanding chemical bonding. Chemical bonding occurs when atoms share or transfer electrons. There are three types of chemical bonds: ionic, covalent, and metallic. Electronegativity is a measure of an atom’s ability to attract electrons in a bond. The Fundamentals of Atomic Structure Atomic structure refers to the composition of an atom, which consists of subatomic particles such as protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons determines the element’s atomic number, while the sum of protons and neutrons gives the atomic mass. The periodic table is a tabular arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. It is a fundamental tool...
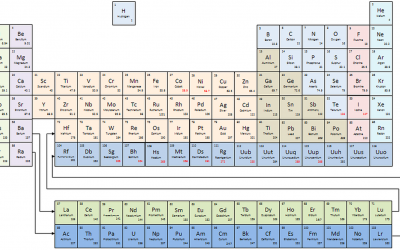
The Periodic Table of Elements
1 H hydrogen1.008 2 He helium4.0026 3 Li lithium6.94 4 Be beryllium9.0122 5 B boron10.81 6 C carbon12.011 7 N nitrogen14.007 8 O oxygen15.999 9 F fluorine18.998 10 Ne neon20.180 11 Na sodium22.990 12 Mg magnesium24.305 13 Al aluminium26.982 14 Si silicon28.085 15 P phosphorus30.974 16 S sulphur32.06 17 Cl chlorine35.45 18 Ar argon39.948 19 K potassium39.098 20 Ca calcium40.078 21 Sc scandium44.956 22 Ti titanium47.867 23 V vanadium50.942 24 Cr chromium51.996 25 Mn manganese54.938 26 Fe iron55.845 27 Co cobalt58.933 28 Ni nickel58.693 29 Cu copper63.546 30 Zn zinc65.38 31 Ga gallium69.723 32 Ge germanium72.63 33 As arsenic74.922 34 Se selenium78.96 35 Br bromine79.904 36 Kr krypton83.798 37 Rb rubidium85.468 38 Sr Strontium87.62 39 Y Yttrium88.906 40 Zr zirconium91.224 41 Nb niobium92.906 42 Mo molybdenum95.96 43 Tc technetium[97.91] 44 Ru ruthenium101.07 45 Rh rhodium102.91 46 Pd palladium106.42 47 Ag silver107.87 48 Cd cadmium112.41 49 In indium114.82 50 Sn tin118.71 51 Sb antimony121.76 52 Te tellurium127.60 53 I iodine126.90 54 Xe xenon131.29 55 Cs cesium132.91 56 Ba barium137.33 72 Hf hafnium178.49 73 Ta tantalum180.95 74 W tungsten183.84 75 Re rhenium186.21 76 Os osmium190.23 77 Ir iridium192.22 78 Pt platinum195.08 79 Au gold196.97 80 Hg mercury200.59 81 Tl thallium204.38 82 Pb lead207.2 83 Bi bismuth208.98 84 Po polonium[208.98] 85 At astatine[209.99] 86 Rn radon[222.02] 87 Fr francium[223.02] 88 Ra radium[226.03] 104 Rf rutherfordium[265.12] 105 Db dubnium[268.13] 106 Sg seaborgium[271.13] 107 Bh bohrium[270] 108 Hs hassium[277.15] 109 Mt meitnerium[276.15] 110 Ds darmstadtium[281.16] 111 Rg roentgenium[280.16] 112 Cn copernicium[285.17] 113 Nh Nihonium[284.18] 114 Fl flerovium[289.19] 115 Mc moscovium[288.19] 116 Lv livermorium[293] 117 Ts tennessine[294] 118 Og oganesson[294] 57 La lanthanum138.91 58 Ce...
Revolutionizing Industries with Nanomaterials: A Look into the Future of British Innovation
Nanomaterials are materials that have been engineered at the nanoscale, typically between 1 and 100 nanometers. At this scale, materials exhibit unique properties and behaviors that differ from their bulk counterparts. Nanomaterials have become increasingly important in various industries due to their ability to enhance performance, improve efficiency, and enable new functionalities. This article will provide an overview of nanomaterials and their revolutionary technology. Summary Nanomaterials are a revolutionary technology with applications in various industries. In the automotive industry, nanomaterials can improve performance and efficiency. Nanomaterials can enhance drug delivery and medical imaging in healthcare. Advancements in solar cells and batteries are possible with nanomaterials in energy. Nanomaterials can improve durability and sustainability in construction. Nanomaterials in the Automotive Industry The automotive industry has embraced the use of nanomaterials to improve the performance, efficiency, and safety of vehicles. One of the key benefits of using nanomaterials in cars is their lightweight nature, which can help reduce fuel consumption and emissions. For example, carbon nanotubes can be used to reinforce composite materials, making them stronger and lighter. This allows for the production of lighter vehicles that require less energy to operate. In addition to weight reduction, nanomaterials can also enhance the mechanical properties of automotive components. For instance, nanoparticles can be added to coatings and paints to improve scratch resistance and durability. Nanoceramic coatings can also be applied to engine components to reduce friction and wear, leading to improved fuel efficiency and longer engine life. Nanomaterials in Healthcare Nanomaterials have revolutionized the healthcare industry by enabling advancements in drug delivery systems and medical imaging technologies. The small size and large...
The Menace of Environmental Pollutants: A Call for Action in Protecting Our Planet
Environmental pollution is a pressing issue that affects the health of humans and the planet. Pollution refers to the introduction of harmful substances or contaminants into the environment, which can have detrimental effects on living organisms and ecosystems. It is a global problem that requires immediate attention and action. The importance of addressing pollution cannot be overstated. Pollution has far-reaching consequences for both human health and the environment. It can lead to a wide range of health problems, including respiratory diseases, cardiovascular diseases, and cancer. Additionally, pollution can disrupt ecosystems, harm wildlife, and contribute to climate change. Therefore, it is crucial to take steps to reduce pollution in order to protect the health of humans and the planet. Summary Environmental pollutants pose a significant threat to our planet and its inhabitants. Pollution has a detrimental impact on human health, wildlife, and ecosystems. The sources of environmental pollution include both human activities and natural causes. Governments and corporations have a responsibility to address pollution through sustainable development practices. Public education and awareness, as well as individual actions, are crucial in reducing pollution and protecting our planet. The Impact of Pollution on Human Health Pollution has a significant impact on human health, causing a wide range of illnesses and diseases. Air pollution, for example, is a major contributor to respiratory problems such as asthma, bronchitis, and chronic obstructive pulmonary disease (COPD). The inhalation of pollutants such as particulate matter and toxic gases can damage the respiratory system and lead to long-term health issues. Water pollution is another major concern for human health. Contaminated water sources can lead to waterborne diseases such...