Fractional Distillation is the process used to separate a liquid mixture into its component parts or in some cases individual elements.
What is Fractional Distillation
The fractioning chamber or fractioning column
Fractional Distillation of Air
Fractional distillation of crude oil
Fractional Distillation in a lab
What is Fractional Distillation
This is a process used to separate a liquid mixture into its component parts or in some cases individual elements. A common method for this is to heat the mixture or compound causing the components to evaporate. The mixture is heated in a large container with several condensing ‘plates’ at different heights; as the mixture heats up and evaporates the various components condense at different temperatures. As the container is heated from the bottom the components that are less volatile and have a high boiling point are separated at the bottom and those that are more volatile and a lower boiling point are separated at the top.

This process is commonly used in the extraction of petrol, diesel etc from crude oil and takes place in the fractioning chamber/column.
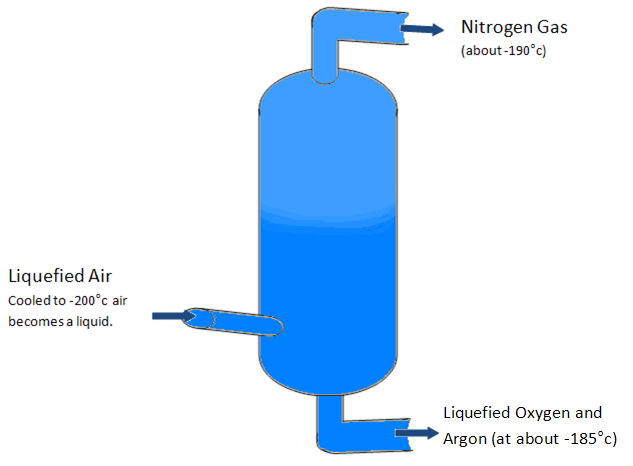
An exception to this type of fractional distillation is the extraction of Nitrogen from air. This process involves cooling atmosphere and allowing the nitrogen to boil off as nitrogen gas, which it does at a higher temperature in the top of the chamber.
Image of an Italian double-effect distillation plant for recovery of solvent, used for the treatment of crude oil
![]() Image by Luigi Chiesa and released under Creative Commons Attribution and ShareAlike License.
Image by Luigi Chiesa and released under Creative Commons Attribution and ShareAlike License.
The fractioning chamber or fractioning column
The fractioning column or chamber (also known as a fractionating column/chamber) is an essential piece of equipment used for the process of fractional distillation (although fractional distillation of air to get nitrogen is possible with a simple tank or chamber). It is basically a cylinder made of glass or metal which normally has an outer shell to insulate against heat loss. Either the bottom of the chamber itself is heated or a heated substance is passed into the bottom of the chamber. The fractionating column has ‘plates’ running through it at different levels. These plates have holes which allow gas and vapours to pass through. Above the holes are concaved lids that force the gas or vapour to hit the topside of the plate and condense into a liquid. As the liquid forms a layer on the top side of the plate, this layer acts as a filter improving the condensation of the passing vapours or gases. The condensed liquid (or condensate) contains less volatile substances than the vapour (volatile substances are substances that are likely to evaporate at lower temperatures), as the volatile substances evaporate and pass the heat up to the plate at the next level in the chamber.
As the chamber is hotter at the bottom with the heat decreasing up the chamber, the most volatile fraction of the initial compound (the fraction with the lowest boiling point) is transported to the top level of the chamber. Different substances or fractions with higher boiling points condense as liquids on the plates below, those with the highest boiling point and least volatile at the bottom and the most volatile at the top. These different substances can be siphoned of the plates and in the case of fractional distillation of crude oil many substances from asphalt to petrol and diesel are extracted.
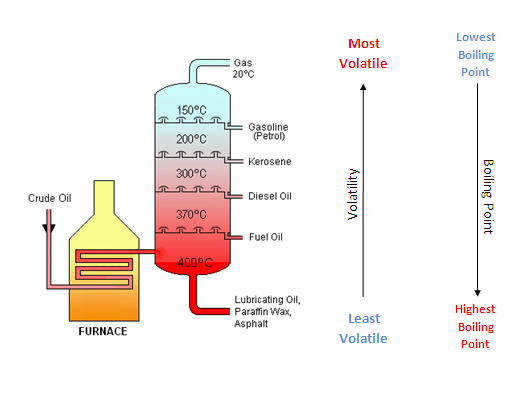
Original diagram drawn by Theresa knott (for original diagram see fractional distillation of crude oil) and adapted by Keir Chapman for Earth Site.
This is a diagram of a typical, atmospheric pressure crude oil distillation tower used in petroleum refining (oil refineries). Released under Creative Commons Attribution and ShareAlike License.
Fractional Distillation of Air
This is the primary source of nitrogen gas and is obtained from cooling the atmosphere or air to -200°c so it becomes a liquid. The Liquefied air is then pumped into a chamber where the temperature increases by several degrease. When the air reaches -190°c the nitrogen in the air boils and becomes a gas once more separating into the top chamber. The remaining liquid is a mixture of Liquid oxygen with a small amount of liquid argon. These elements are then separated in a second chamber through the same process.
Fractional distillation of crude oil
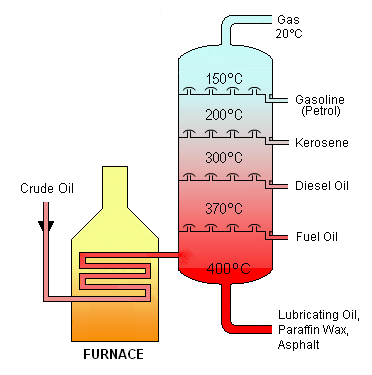
Diagram drawn by Theresa knott. This is a diagram of a typical, atmospheric pressure crude oil distillation tower used in petroleum refining (oil refineries). Released under Creative Commons Attribution and ShareAlike License.
The process takes advantage of the fact that the longer the carbon chain, the higher the temperature at which the compounds will boil. Crude oil is turned into gas through heating in the furnace. The gas passes through a distillation column where the temperature decreases with height. As the gas or vapour reaches a certain height in the chamber it cools and some of the compounds condense into liquids at these temperatures. At this point the liquids are collected on ‘condensing trays’ and are siphoned off through pipes. Eventually all that is left is the petroleum gas which passes through a pipe at the top of the condensing unit.
Fractional Distillation in a lab
Extraction through fractional distillation is possible in any lab using common apparatus. The required equipment is as follows:
- A heat source such as a Bunsen burner.
- A round bottomed flask (which gives a larger surface area for even heating)
- A fractionating column
- A thermometer to closely observe the temperature in the fractioning column.
- Condenser (has cold water running through it to cool the gas or vapours)
- Flat bottomed beaker to catch the condensate.
- Clamps to hold apparatus in place.
Diagram
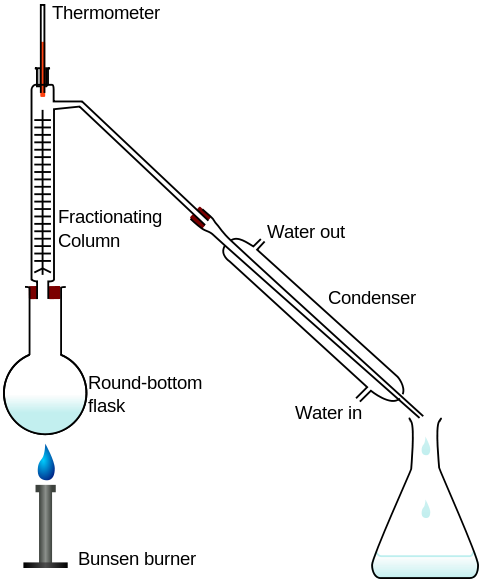
Diagram, drawn by John Kershaw, derived from a PNG drawn by Theresa Knott. This diagram was created with Inkscape.
![]() Released under Creative commons Attribution and ShareAlike License.
Released under Creative commons Attribution and ShareAlike License.
Method
The solution is placed in the round-bottomed flask and heated. The temperature in the fractioning column is observed from the thermometer and can be controlled by reducing the flame on the Bunsen burner. The temperature at the top of the fractioning chamber needs to reach the boiling point of the most volatile fraction of the solution required. When the temperature reaches this point care needs to be taken to keep this temperature constant until solution is separated. The more volatile fraction will be boiled off as vapour. This vapour will travel through the tube at the top of the fractioning column and down to the condensing unit. As the cold water passes through the outside chamber of the condenser it cools the vapours running through the centre tube. These vapours condense into liquid or condensate which is collected in the flat-bottomed flask. The remaining liquid in the round-bottomed flask will contain the less volatile fraction of the original solution.