Nitrogen is the most abundant gas in our atmosphere making up approximately 78% with 21% oxygen, 0.93% argon, and rest is made up other various gases.
Atmospheric Nitrogen Causing Aurora

Nitrogen (from the Latin words “nitron” meaning native soda and “gene” meaning from)

Classification: Non-Metallic
Atomic Mass: 14.0067 g/mol
Density: 1.251g/l
Colour: No Colour
Boiling Point: 77.36K (-195.79°C)
Melting Point: 63.05K (-210.1°C)
Critical Temperature: 126.2K (-146.9°C)
Discovery of Nitrogen
Discovered by Scottish Physician Daniel Rutherford in 1772. Rutherford isolated nitrogen when he was studying carbon dioxide.
Sources
Nitrogen is the most abundant gas in our atmosphere making up approximately 78% with 21% oxygen, 0.93% argon, 0.039% carbon dioxide and 0.031% makes up other various gases.
Nitrogen is obtained from the fractional distillation of liquefied air. This process requires the air to be cooled to -200°c so it becomes a liquid. The Liquefied air is then pumped into a chamber where the temperature increases by several degrease. When the air reaches -190°c the nitrogen in the air boils and becomes a gas once more separating into the top chamber. The remaining liquid is a mixture of Liquid oxygen with a small amount of liquid argon. These two are then separated in a second chamber through the same process.
Uses

Liquid Nitrogen is nitrogen gas that has been cooled sufficiently enough for it to revert to its liquid state, Nitrogen becomes a liquid (at normal atmospheric pressure) between -210o C to -196o C (63 K to 77.2 K or -346°F to -320.44°F). It is used in cryogenics which is the study of the property of materials at very low temperature. Liquid nitrogen is the most common substance used to reduce temperatures in cryogenics as it is legal and easily accessible.
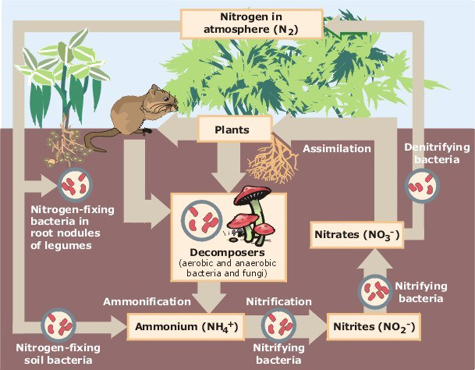
The nitrogen cycle is another example of nature recycling non-renewable resources. Nitrogen is an essential nutrient for growth forming proteins and amino acids which build plant and animal life. Nitrogen is fixed by bacteria into compounds making it accessible to plants. It is taken up through the root and are converted into proteins and various plant cells. When eaten the proteins are converted into animal proteins and tissue. Travelling from animal to animal through consumption some nitrates are returned to the soil through death and decomposition as well as through excretion on waste matter. For more detailed information click on the link above.
The colours of the Aurora Borealis or the northern lights are caused by cosmic rays exciting the Oxygen and Nitrogen in the upper atmosphere causing them to produce photons of various colours. The red and green colours are produced by oxygen while the blue and crimson are caused by excited nitrogen particles
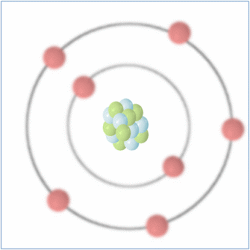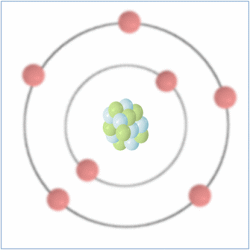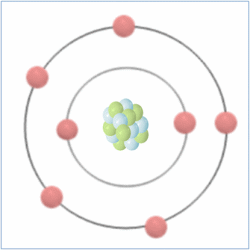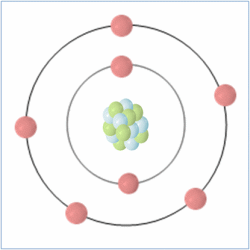
Shell Structure
Protons = 7
Neutrons = 7
Electrons= 7
|
|
s |
p |
d |
f |
|
1 |
2 |
|
|
|
|
2 |
2 |
3 |
|
|
|
3 |
|
|
|
|
|
4 |
|
|
|
|
|
5 |
|
|
|
|
|
6 |
|
|
|
|
|
7 |
|
|
|
|
Absorption Lines

Emission Lines