🧪 Introduction to Chemistry
Unlocking the Secrets of Matter and Change
Chemistry is the science of matter—what it’s made of, how it behaves, and how it changes. It explores everything from the tiniest atoms and molecules to the vast chemical reactions that fuel stars, power engines, and sustain life itself. Often called the “central science,” chemistry connects physics with biology, medicine, geology, environmental science, and even engineering.
At its core, chemistry seeks to answer questions like:
-
What is this substance made of?
-
How does it interact with other substances?
-
Why do some materials burn, rust, or dissolve?
-
How can we create new materials, medicines, or fuels?
From the food we eat to the air we breathe, from cleaning products to smartphones, chemistry is everywhere. It helps explain natural phenomena like fire, digestion, and photosynthesis, while also driving innovations in technology, health, and sustainability.
By studying chemistry, we gain a deeper understanding of the world at a molecular level—and the tools to change it for the better.
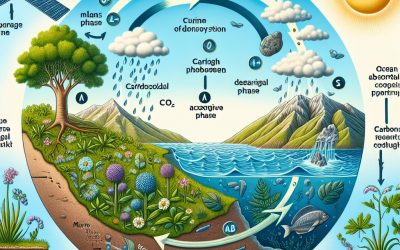
The Carbon Cycle
Plants to animals Fossil Fuels Calcium Carbonate Our planet has got many systems or cycles in place that allow for the re-use of materials which are vital for life. These include nitrogen, water and carbon. The recycling of these vital materials helps support the continued existence of life that would otherwise end. We, as all living things, do not simply use this cycle but are a part of it. Plants to animals...
Beryllium: Periodic Table, Uses, Occupational Safety and Health
Beryllium: Periodic Table, Uses, Occupational Safety and Health Basic Information Discovery Sources Uses Use in Telescopes Use in Satellites Cell Structure Absorption Lines Emission Lines Beryllium (named after the mineral, beryl, that it was originally isolated from) Classification: Alkali earth metal Atomic Mass: 9.012182 (3) g/mol Density: 1.85g/cm3 Colour: grey Boiling Point: 2742K (2469°C) Melting Point: 1560K (1287°C) Beryllium: The Lightweight Power Metal Shaping Modern Industry and Safety Standards What makes beryllium so important in science, industry, and worker safety? Beryllium might not be a household name, but this light metal with atomic number 4 holds a significant place on the periodic table and in high-tech industries. Whether you’re flying on an aeroplane, undergoing an X-ray, or benefiting from advanced communication technologies, beryllium plays a hidden yet vital role. This article explores the fascinating properties of beryllium, its various applications—from alloys to nuclear reactors—and the critical occupational safety and health concerns related to its use. If you’re curious about how this relatively rare element shapes modern life, this post is a must-read. Article Outline 1. What Is Beryllium and Why Does It Matter? 2. Where Is Beryllium Found and How Is It Extracted? 3. What Are the Unique Chemical Properties of Beryllium? 4. How Is Beryllium Used in Alloys and Why? 5. How Does Beryllium Help in X-ray Technology? 6. What Role Does Beryllium Play in Nuclear Applications? 7. What Are the Health Risks of Exposure to Beryllium? 8. How Does OSHA Regulate Beryllium Exposure? 9. Why Is Beryllium So Valuable in Aerospace and Defence? 10. What Should You Know About the Future of Beryllium Use?...