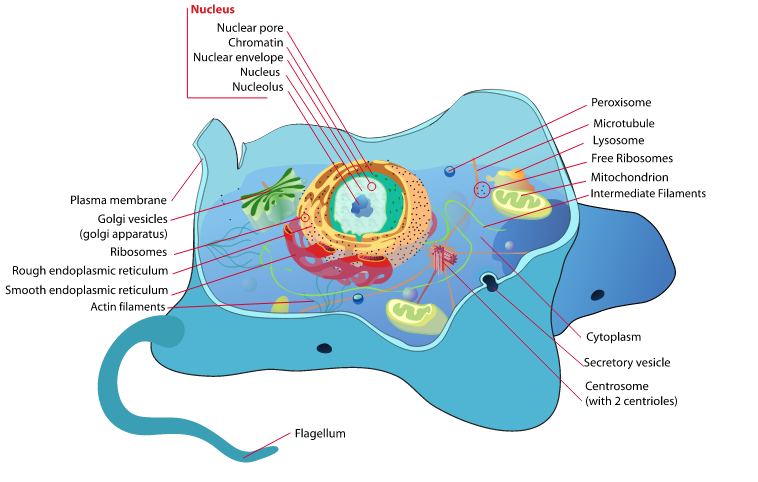
Lysosomes are membrane-bound organelles found in animal cells that play a crucial role in cellular waste management and recycling. They were first discovered by Belgian cytologist Christian de Duve in the 1950s, who coined the term “lysosome” to describe these structures. Lysosomes contain a variety of enzymes that are responsible for breaking down and recycling cellular waste materials, such as proteins, lipids, and carbohydrates.
The importance of lysosomes in cellular function cannot be overstated. Without lysosomes, cells would not be able to efficiently remove and recycle waste materials, leading to a buildup of toxic substances and impaired cellular function. Lysosomes also play a role in other cellular processes, such as cell signaling, nutrient sensing, and immune response.
Key Takeaways
- Lysosomes are the cellular recycling center responsible for breaking down and recycling cellular waste.
- Lysosomes are composed of a membrane-bound sac filled with enzymes that degrade and recycle cellular waste.
- Lysosomes play a critical role in cellular waste management, preventing the accumulation of toxic waste products.
- Lysosomal enzymes are the key players in degradation and recycling, breaking down a wide range of cellular waste products.
- Lysosomal autophagy is the process of self-eating, where lysosomes break down and recycle damaged organelles and proteins.
The Structure and Function of Lysosomes: Understanding the Basics
Lysosomes are small spherical organelles that are surrounded by a single membrane. They contain a variety of hydrolytic enzymes that are responsible for breaking down macromolecules into smaller components that can be recycled by the cell. The lysosomal membrane is composed of lipids and proteins that help maintain the integrity of the organelle and regulate the transport of molecules into and out of the lysosome.
The function of lysosomes can be divided into two main categories: intracellular digestion and autophagy. In intracellular digestion, lysosomes fuse with endocytic vesicles that contain materials taken up by the cell through processes such as phagocytosis or pinocytosis. The enzymes within the lysosome then break down these materials into smaller molecules that can be used by the cell.
Autophagy is another important function of lysosomes. During autophagy, damaged or unnecessary cellular components are engulfed by a double-membrane structure called an autophagosome. The autophagosome then fuses with a lysosome, allowing the contents to be degraded and recycled. This process is crucial for maintaining cellular homeostasis and preventing the accumulation of damaged proteins and organelles.
The Role of Lysosomes in Cellular Waste Management: A Critical Function
Cellular waste management is a vital process that ensures the proper functioning of cells. It involves the removal and recycling of damaged or unnecessary cellular components, as well as the breakdown of macromolecules into smaller molecules that can be used for energy or building blocks.
Lysosomes play a central role in cellular waste management. They are responsible for the degradation of various cellular components, including proteins, lipids, carbohydrates, and nucleic acids. The enzymes within lysosomes break down these macromolecules into their constituent parts, which can then be recycled by the cell.
One example of lysosomal degradation is the breakdown of proteins. Proteins that are no longer needed or have become damaged are targeted for degradation by a process called ubiquitination. These proteins are then recognized by lysosomes and transported to the organelle for degradation. The enzymes within lysosomes break down the proteins into amino acids, which can be used by the cell to build new proteins.
Lysosomal Enzymes: The Key Players in Degradation and Recycling
| Lysosomal Enzymes | Function | Related Diseases |
|---|---|---|
| Proteases | Degrade proteins into amino acids | Multiple sclerosis, Alzheimer’s disease |
| Lipases | Degrade lipids into fatty acids and glycerol | Gaucher’s disease, Niemann-Pick disease |
| Glycosidases | Degrade carbohydrates into simple sugars | Tay-Sachs disease, Pompe disease |
| Nucleases | Degrade nucleic acids into nucleotides | Lysosomal storage diseases |
Lysosomal enzymes are a diverse group of hydrolytic enzymes that are responsible for breaking down macromolecules into smaller components. There are several types of lysosomal enzymes, including proteases, lipases, carbohydrases, and nucleases.
Proteases are enzymes that break down proteins into amino acids. They play a crucial role in the degradation of damaged or unnecessary proteins, as well as in the turnover of cellular components. Lipases are enzymes that break down lipids into fatty acids and glycerol. They are involved in the breakdown of lipids for energy production and the recycling of cellular membranes.
Carbohydrases are enzymes that break down carbohydrates into simple sugars. They are responsible for the degradation of glycogen, a storage form of glucose, as well as other complex carbohydrates. Nucleases are enzymes that break down nucleic acids, such as DNA and RNA, into nucleotides. They play a role in the turnover of nucleic acids and the recycling of cellular components.
The importance of lysosomal enzymes in cellular function cannot be overstated. Without these enzymes, cells would not be able to efficiently degrade and recycle macromolecules, leading to a buildup of waste materials and impaired cellular function.
Lysosomal Autophagy: The Process of Self-Eating
Autophagy is a cellular process that involves the degradation and recycling of damaged or unnecessary cellular components. It is an essential mechanism for maintaining cellular homeostasis and preventing the accumulation of toxic substances.
During autophagy, damaged or unnecessary cellular components are engulfed by a double-membrane structure called an autophagosome. The autophagosome then fuses with a lysosome, allowing the contents to be degraded and recycled. This process is crucial for maintaining cellular homeostasis and preventing the accumulation of damaged proteins and organelles.
Autophagy plays a role in various physiological processes, including development, aging, and immune response. It is also involved in the pathogenesis of several diseases, including cancer, neurodegenerative disorders, and metabolic diseases.
Lysosomal Storage Diseases: When the Recycling Center Fails
Lysosomal storage diseases are a group of inherited metabolic disorders that result from defects in lysosomal function. These disorders are characterized by the accumulation of undegraded macromolecules within lysosomes, leading to cellular dysfunction and tissue damage.
There are more than 50 different lysosomal storage diseases, each caused by a specific genetic mutation that affects the production or function of lysosomal enzymes. Examples of lysosomal storage diseases include Gaucher disease, Tay-Sachs disease, and Pompe disease.
The symptoms of lysosomal storage diseases vary depending on the specific disorder and the organs affected. Common symptoms include developmental delay, neurologic abnormalities, skeletal abnormalities, and organ dysfunction. The severity of symptoms can range from mild to severe, and the age of onset can vary from infancy to adulthood.
Treatment options for lysosomal storage diseases are limited and often focus on managing symptoms and slowing disease progression. Enzyme replacement therapy, substrate reduction therapy, and gene therapy are some of the approaches that have been used to treat lysosomal storage diseases.
Lysosomes and Disease: Implications for Therapeutic Interventions
Lysosomes play a critical role in the pathogenesis of various diseases, including cancer, neurodegenerative disorders, and metabolic diseases. Dysregulation of lysosomal function can lead to the accumulation of toxic substances, impaired cellular function, and tissue damage.
Understanding the role of lysosomes in disease has led to the development of therapeutic interventions that target these organelles. One example is the use of lysosome-targeted drugs for the treatment of cancer. These drugs are designed to selectively accumulate in lysosomes and disrupt their function, leading to cell death.
Another example is the use of lysosome-targeted therapies for the treatment of neurodegenerative disorders. These therapies aim to enhance lysosomal function and promote the clearance of toxic protein aggregates that are characteristic of these diseases.
Lysosomal Trafficking and Secretion: Beyond Waste Management
In addition to their role in waste management and recycling, lysosomes also play a role in other cellular processes, such as cell signaling, nutrient sensing, and immune response. Lysosomes are involved in the trafficking and secretion of various molecules, including growth factors, cytokines, and neurotransmitters.
Lysosomal trafficking refers to the movement of lysosomes within the cell. Lysosomes can move along microtubules and interact with other organelles, such as the endoplasmic reticulum and the Golgi apparatus. This dynamic movement allows lysosomes to respond to cellular cues and participate in various cellular processes.
Lysosomal secretion refers to the release of lysosomal contents into the extracellular space. Lysosomes can fuse with the plasma membrane and release their contents, including enzymes and other molecules. This process is involved in cell signaling, immune response, and tissue remodeling.
Emerging Research on Lysosomes: New Insights and Discoveries
Research on lysosomes is a rapidly evolving field, with new insights and discoveries being made on a regular basis. Recent studies have shed light on the role of lysosomes in various cellular processes, as well as their involvement in disease pathogenesis.
One area of emerging research is the role of lysosomes in cellular metabolism. Lysosomes have been found to play a role in nutrient sensing and energy homeostasis, and dysregulation of lysosomal function has been implicated in metabolic diseases such as obesity and diabetes.
Another area of emerging research is the role of lysosomes in immune response. Lysosomes are involved in antigen presentation, phagocytosis, and autophagy, all of which are crucial for immune function. Dysregulation of lysosomal function has been implicated in autoimmune diseases and infectious diseases.
Future Directions in Lysosome Research: Advancing Our Understanding of Cellular Waste Management
Lysosome research holds great promise for advancing our understanding of cellular waste management and its implications for human health and disease management. Continued research in this field will help uncover new insights into the structure and function of lysosomes, as well as their role in cellular processes.
Future directions in lysosome research include the development of new techniques for studying lysosomes, such as advanced imaging techniques and proteomic approaches. These techniques will allow researchers to visualize lysosomes in real-time and identify the proteins and enzymes that are present within these organelles.
Another future direction in lysosome research is the development of novel therapeutic interventions that target lysosomes. This includes the development of lysosome-targeted drugs for the treatment of cancer, neurodegenerative disorders, and metabolic diseases. It also includes the development of gene therapies that aim to correct genetic mutations that affect lysosomal function.
In conclusion, lysosomes are a critical component of cellular waste management and recycling. They play a crucial role in degrading and recycling macromolecules, as well as in maintaining cellular homeostasis. Lysosomes are involved in various cellular processes, including intracellular digestion, autophagy, and lysosomal trafficking and secretion. Dysregulation of lysosomal function can lead to the development of lysosomal storage diseases and other disorders. Continued research in this field will help advance our understanding of cellular waste management and its implications for human health and disease management.
FAQs
What is a lysosome?
A lysosome is a membrane-bound organelle found in animal cells that contains digestive enzymes capable of breaking down various biomolecules.
What is the function of a lysosome?
The primary function of a lysosome is to break down and recycle cellular waste materials, including proteins, lipids, and carbohydrates. It also plays a role in the destruction of invading bacteria and viruses.
How does a lysosome work?
Lysosomes contain hydrolytic enzymes that are capable of breaking down various biomolecules. The enzymes are activated when the lysosome fuses with a vesicle containing the material to be digested. The resulting breakdown products are then released into the cytoplasm for reuse.
What happens if lysosomes malfunction?
If lysosomes malfunction, they can cause a variety of diseases, including lysosomal storage disorders, which are characterized by the accumulation of undigested materials within the lysosome. This can lead to cell damage and organ dysfunction.
What is autophagy?
Autophagy is a process by which cells break down and recycle their own components. This process is mediated by lysosomes, which engulf and digest the targeted material. Autophagy plays a critical role in maintaining cellular homeostasis and preventing the accumulation of damaged or dysfunctional components.