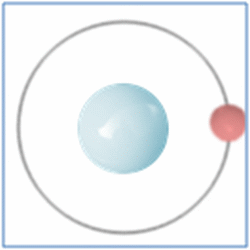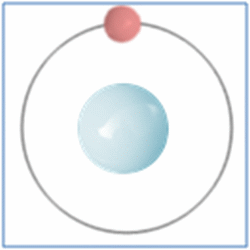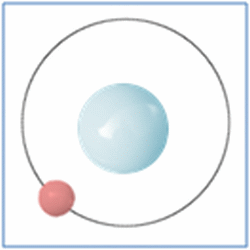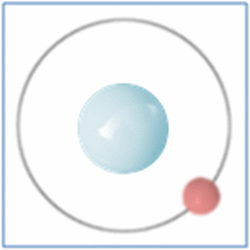
Hydrogen is the lightest and most abundant element in the universe.
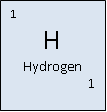
Hydrogen (from the Greek hudor (meaning water) and gennan (meaning generate)

Classification: Non-metallic
Atomic Mass: 1.00794 g/mol
Density: 0.08988g/cm3
Colour: None
Boiling Point: 20.268K (-252.87°C
Melting Point: 14.01K (-259.14°C)
Critical Temperature: 33K (-240°C)
Discovery
Hydrogen was discovered in 1766 by English physicist Henry Cavendish. Cavendish conducted numerous experiments and eventually identified that hydrogen was a unique gas with its own set of properties. Fast forward to today, and the significance of hydrogen is more apparent than ever. This little molecule holds incredible potential as a clean and renewable energy source. Scientists and researchers worldwide are tirelessly working to harness its power and overcome some of the current challenges that come with its production and storage.
Sources
Hydrogen is the most abundant element in the universe with nearly 90% of all visible atoms being hydrogen. The first atoms ever created after the Big Bang would have been that of hydrogen and helium which eventually culminated into stars. Due to the intense heat and pressure within the stars the hydrogen is in a state known as plasma and nuclear fission turns the hydrogen atoms into Helium, the next most abundant element.
On earth Hydogen is most abundant in the sea where it has been mixed with oxygen to create water.
Uses
Hydrogen is used in the production of ammonia (NH3), ethanol (alcahol(C2H5OH)) and hydrogen Chloride (HCL) among many other uses.
Hydrogen has got two stable isotopes; one is called Deuterium with one proton and one neutron and Tritium with one proton and two neutrons.
On the 1st of November 1952 the United States of America tested their new Hydrogen bomb which greatly exceeded their expectations.
The early Hydrogen bombs fused hydrogen nuclei (nuclear fusion) unleashing terrific amounts of energy, even more than that of the atom bomb which used nuclear fission to split the atom. Modern Thermonuclear weapons use hydrogen fission as a trigger to trigger a secondary, more powerful nuclear explosion.
Airships were widely used pre 1940 in a variety of applications. Early versions used a Hydrogen filled pocket to produce lift as it is lighter than the surrounding atmosphere. The use of hydrogen was unsafe due to its combustibility which proved fatal, famously in the Hindenburg disaster.
Airships now use non-flammable helium which has become less expensive since the discovery of large deposits in America.
A more modern use for Hydrogen has been in fuel cells which can power cars and even buses instead of fossil fuels. The cells work by converting the energy from a chemical reaction into electricity. Usually hydrogen and oxygen are used which produces water vapour as a by product.
The Power of Hydrogen
- Clean and Efficient Energy: When used as a fuel, hydrogen produces only water vapor and emits no greenhouse gases or pollutants. Its combustion in fuel cells generates electricity, providing a clean and efficient energy source for various applications.
- Renewable Energy Storage: One of the major obstacles for renewable energy sources like solar and wind is their intermittent nature. Hydrogen offers a solution by serving as a means of storing surplus energy during peak production times, which can then be converted back to electricity when needed.
- Transportation Revolution: Hydrogen fuel cell vehicles have garnered significant attention due to their long ranges and fast refueling times. These vehicles use hydrogen to create electricity, eliminating the need for fossil fuels and reducing greenhouse gas emissions.
- Decentralized Energy: With the help of hydrogen, communities and businesses can develop their own localized energy systems. This decentralization promotes energy resilience and reduces dependence on centralized power grids.
##Overcoming Challenges
While hydrogen holds great promise, there are several challenges that need to be addressed for its widespread adoption: - Production: The majority of hydrogen produced today comes from fossil fuels, making it a carbon-intensive process. However, advancements in renewable energy technologies can pave the way for greener hydrogen production methods.
- Storage and Distribution: Hydrogen has low energy density, requiring efficient storage and distribution systems. Solutions like high-pressure tanks, liquid hydrogen, and metal hydrides are being explored to overcome these challenges.
- Infrastructure: For hydrogen to become a widely available fuel, a comprehensive infrastructure needs to be established. This includes hydrogen filling stations, pipelines, and storage facilities.
The Future of Hydrogen
- Hydrogen Economy: Many experts envision a future where hydrogen plays a central role in our energy systems. This hydrogen economy would involve widespread use of hydrogen for power generation, heating, transportation, and industrial processes.
- Integration with Renewables: As the share of renewable energy in our overall energy mix grows, hydrogen can act as a key enabler. It can absorb excess renewable energy, balance the grid, and provide a sustainable source of power during times of low renewable generation.
- Innovation and Collaboration: Governments, industries, and researchers are collaborating to accelerate the development and deployment of hydrogen technologies. Investments in research, development, and infrastructure are crucial for driving innovation and making hydrogen a mainstream energy option.
##Conclusion
Hydrogen holds enormous potential to revolutionize our energy systems and combat climate change. Its clean and efficient nature, coupled with its versatility and ability to store renewable energy, make it a frontrunner in the race towards a sustainable future. Overcoming the challenges associated with production, storage, and infrastructure is essential for unlocking the full potential of hydrogen. Let us embrace this fuel of the future and pave the way to a cleaner and greener world.
Shell Structure
Protons = 1
Neutrons =0
Electrons=1
|
s |
p |
d |
f |
|
|
1 |
1 |
|||
|
2 |
||||
|
3 |
||||
|
4 |
||||
|
5 |
||||
|
6 |
||||
|
7 |
Absorption Lines

Emission Lines

See Also:
The Power of Hydrogen: Exploring the Potential of H2 as a Clean Energy Source