Lithium is a soft silver-white metal with a range of applications including medication,batteries and fireworks.

Lithium (from the Greek Lithos meaning Stone)

Classification: Alkali metal
Atomic Mass: [6.941 (2)] g/mol
Density: 0.534g/cm3
Colour: metallic silver or greyish white
Boiling Point: 1615K (1342°C)
Melting Point: 453.69K (180.54°C)
Critical Temperature:3223K (2950°C)
Discovery of Lithium
Lithium (3Li) was first discovered in the mineral petalite (LiAlSi4O10) by Johann Arfvedson in Sweden 1817.
Sources
It is harvested from compounds found in most igneous rocks, clay, brine sources and through electrolysis of compounds like lithium chloride.
3Li is a highly reactive and flammable metal like all the alkali metals and is only found in nature as part of a compound. A pure sample is a metallic silver metal however this very quickly changes into a dull whitish grey or black colour as it reacts with moisture or oxygen in the air. 3Li is the only alkali metal that reacts with nitrogen. To prevent reactions with other elements it is usually stored in mineral oil which acts as a barrier or protective seal.
Uses
In medicine 3Li is used to regulate the sodium levels in the muscles and nerves reducing the effects of mania in those that suffer from manic depression or manic episodes.

3Li helps reduce some of the symptoms associated with the illness such as hyperactivity, insomnia, aggression and other behavioural problems that may stem from the illness. 3Li is normally administered in time release tablets to prevent overdosing and as a last resort or in extreme cases due to the severe side effects connected with this drug. The side effects include nausea, vomiting, drowsiness, muscle weakness, tremor, reduced coordination, blurred vision or ringing in your ears.
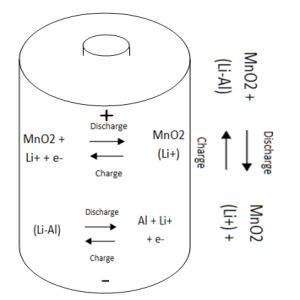
3Li is used in some batteries as the anode and magnese oxide as the cathode, the chemical reaction produces a small charge which is dispersed from the battery. 3Li batteries usually have a longer life than regular alkaline batteries making them ideal for pacemakers and other medical devices. These batteries are more expensive however due to the 3Li required.

When placed in a flame 3Li produces a red flame and as it is flammable it is ideal for flares or fireworks. It is also used in various compuonds in rocket fuel and as torpedo propellant.
3Li is also used in the form of lithium-peroxide or lithium-hydroxide to purify the air in submarines and spacecraft as it is efficient in absorbing carbon dioxide from the atmosphere.
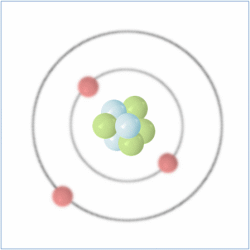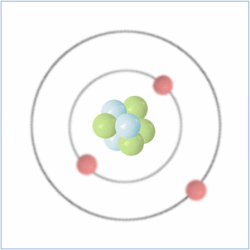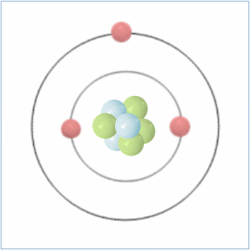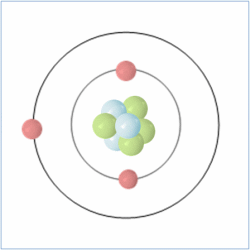
Shell Structure

Protons = 3
Neutrons =4
Electrons=3
|
|
s |
p |
d |
f |
|
1 |
2 |
|
|
|
|
2 |
1 |
|
|
|
|
3 |
|
|
|
|
|
4 |
|
|
|
|
|
5 |
|
|
|
|
|
6 |
|
|
|
|
|
7 |
|
|
|
|
Absorption Lines

Emission Lines