Radioactive dating is a process whereby a person can calculate the age of an object by measuring its rate of radioactive decay.
Isotopes used in Geological Dating
Radioactive Dating with Uranium-238
What is Radioactive Dating?
Radioactive dating is a process whereby a person can calculate the age of an object by measuring its rate of radioactive decay. This is because the rate of decay is consistent with any particular isotope and is not affected by outside environmental influences. A sample of radioactive material will contain a certain amount of the unstable isotope that has not yet decayed into its stable form. By comparing the amount of unstable and stable isotopes we can calculate the age of some materials. In a particular sample of radioactive material it takes a set amount of time for half of the nuclei to succumb to radioactive decay which is called the radioactive half-life.
Carbon Dating
All life forms intake carbon during their lives and a small proportion of that carbon is in the form of carbon-14 (due to cosmic rays). Within the body of living organism the percentage of carbon-14 and carbon-12 meet a certain level which is sustained until its death. At this point no more carbon is ingested and the ‘clock’ starts. The levels of carbon-14 (6C14) start to drop as it decays into a nitrogen ion (7N14) and by knowing the rate of decay you can compare the levels carbon-12 and carbon-14 to calculate the approximate age of the organic material. This method however is only suitable for samples of organic origin within about 50,000 years as samples predating this would contain too small amounts of carbon-14 to accurately measure. It is also argued that amounts of carbon-14 levels may have been different previously due to the amount of cosmic rays making this process inaccurate.
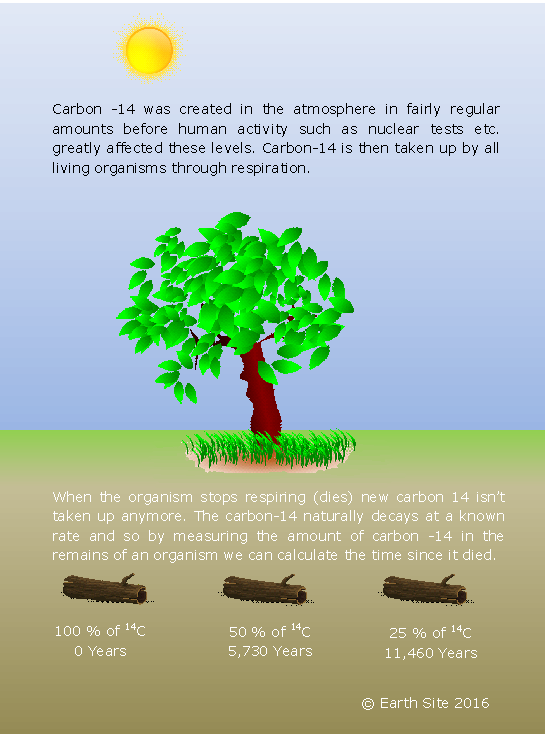
Carbon-14 has a half-life of 5730 years. Carbon-14 is produced in the upper atmosphere when atoms are bombarded with cosmic rays creating highly energetic neutrons. These excited neutrons collide with the Nitrogen in the atmosphere causing one of its protons to be released and producing carbon-14. Carbon-14 is then taken in by the plants in the form of CO2 just like normal carbon-12 and due to the constant creation of the carbon-14 isotopes; the concentration of these types of carbon stays constant. The ratio of carbon on earth is thought to be 99% carbon-12, just under 1% Carbon-13 and 0.0000000001% of carbon-14 with the same ratios found in all living things.
Geological Dating
>
With carbon dating the ‘clock’ starts when the organism dies and it stops up taking carbon, however with radioactive dating of rocks the ‘clock’ begins when the rock is formed. The radioactive isotopes used when dating rocks have incredibly long half-lives such as uranium-238 which has a half-life of about 4.6 billion years. Dating the rocks requires finding out the ratio of the original or ‘parent’ isotope for example Uranium-238 and the stable or ‘daughter’ isotope which in this case would be lead-206.
Isotopes used in Geological Dating
|
Parent isotope (radioactive) |
Half-life (Gy (Billion Years)) |
Daughter isotope (stable) |
|
40K |
1.25 |
40Ar |
|
87Rb |
48.8 |
87Sr |
|
147Sm |
106 |
143Nd |
|
176Lu |
35.9 |
176Hf |
|
187Re |
43 |
187Os |
|
232Th |
14 |
208Pb |
|
235U |
0.704 |
207Pb |
|
238U |
4.47 |
206Pb |
Radioactive Dating with Uranium-238
By knowing the half-life of uranium-238, 4.6 billion years, we can calculate the age of the rock by discovering the ratio of uranium-238 and lead-206.
One obstacle with this method is determining the percentage of the ‘daughter’ isotope (lead-206) which may have originally formed in the rock. In the example case of dating uranium-238 with a daughter isotope of lead we can calculate the amount of lead from original formation. This is because lead has 4 isotopes which have the same ratio wherever lead is found. One of these isotopes, lead-204, is not produced from radioactive decay so by measuring it’s quantity we get a strong indication of the original lead-206 concentration in the rock at the time of formation.
Some radioactive isotopes decay into stable isotopes directly, but some unstable or radioactive isotopes decay into other unstable isotopes many times before becoming stable and these stages are explained in the ‘decay chain’. By using this decay chain and knowing the half-life of all the subsequent isotopes at each stage we can use their ratios to give a more accurate dating method. To calculate these ratios a mass spectrometer is normally used which precisely records the quantities of isotopes in a given sample.
By this method we know that the some rocks formed on the earth date back 4.03 billion years ago.