Beryllium: Periodic Table, Uses, Occupational Safety and Health

Beryllium (named after the mineral, beryl, that it was originally isolated from)

Classification: Alkali earth metal
Atomic Mass: 9.012182 (3) g/mol
Density: 1.85g/cm3
Colour: grey
Boiling Point: 2742K (2469°C)
Melting Point: 1560K (1287°C)
Beryllium: The Lightweight Power Metal Shaping Modern Industry and Safety Standards
What makes beryllium so important in science, industry, and worker safety?
Beryllium might not be a household name, but this light metal with atomic number 4 holds a significant place on the periodic table and in high-tech industries. Whether you’re flying on an aeroplane, undergoing an X-ray, or benefiting from advanced communication technologies, beryllium plays a hidden yet vital role. This article explores the fascinating properties of beryllium, its various applications—from alloys to nuclear reactors—and the critical occupational safety and health concerns related to its use. If you’re curious about how this relatively rare element shapes modern life, this post is a must-read.
Article Outline
1. What Is Beryllium and Why Does It Matter?
2. Where Is Beryllium Found and How Is It Extracted?
3. What Are the Unique Chemical Properties of Beryllium?
4. How Is Beryllium Used in Alloys and Why?
5. How Does Beryllium Help in X-ray Technology?
6. What Role Does Beryllium Play in Nuclear Applications?
7. What Are the Health Risks of Exposure to Beryllium?
8. How Does OSHA Regulate Beryllium Exposure?
9. Why Is Beryllium So Valuable in Aerospace and Defence?
10. What Should You Know About the Future of Beryllium Use?
What Is Beryllium and Why Does It Matter?
Beryllium is a chemical element with the symbol Be and atomic number 4, placing it near the top of the periodic table. As a light metal, it’s known for its high thermal conductivity, strength, and non-magnetic nature. Although it’s a relatively rare element, its impact in industrial and scientific fields is immense.
The element beryllium is found in nature primarily in beryl and bertrandite, both of which are important ores of beryllium. Because of its high melting point and ability to form a tough oxide layer, beryllium metal performs well under extreme conditions, making it indispensable in high-performance settings.
Where Is Beryllium Found and How Is It Extracted?
Beryllium ores are relatively scarce, with beryl and emerald being the most well-known minerals containing beryllium. Another major ore is bertrandite, which is processed through chemical methods to make beryllium in usable forms.
Production of beryllium typically involves the chemical extraction of beryllium hydroxide from bertrandite, followed by its conversion to beryllium metal or beryllium compounds like beryllium fluoride and beryllium chloride. A sample of beryllium is incredibly light yet strong, making it ideal for specialised uses.
What Are the Unique Chemical Properties of Beryllium?
Among all metals, beryllium is unique in its physical properties and chemical properties. It has a low atomic number, a high melting point, and is relatively transparent to X-rays, allowing its widespread use in diagnostic tools.
The oxide film that naturally forms on beryllium’s surface helps it resist corrosion. Beryllium oxidizes slowly at room temperature, thanks to this oxide layer. The element also possesses a hexagonal beryllium crystal structure and releases electrons in a stable manner, contributing to its strength and reactivity.
How Is Beryllium Used in Alloys and Why?
One of the most common applications is in beryllium alloys, especially beryllium copper, which combines the strength of beryllium with the ductility of copper and nickel. These alloys are used in electrical connectors, precision instruments, and aerospace components.
Beryllium alloys are used because they retain high strength even at elevated thermal conditions. A mixture of beryllium with metals like aluminium or magnesium increases resistance to wear and fatigue, making them ideal for components made of beryllium in demanding environments.
How Does Beryllium Help in X-ray Technology?
Beryllium is used in x-ray windows due to its relatively transparent nature to x-rays and low atomic number. This allows X-rays to pass through with minimal interference, which is crucial in both medical diagnostics and industrial imaging.
Thin sheets, known as beryllium foil, serve as effective barriers that let x-rays through while blocking visible light and gases. Because of this, beryllium is also used in vacuum-sealed imaging devices and x-ray detectors.
What Role Does Beryllium Play in Nuclear Applications?
The unique nuclear characteristics of beryllium, particularly its ability to reflect and moderate neutrons, make it essential in nuclear reactors. It can be used to make beryllium neutron reflectors or as a target material for neutron production.
Thanks to its stable beryllium forms, such as refractory beryllium, and properties like a high melting point, beryllium metal is used in radiation environments where other metals might fail. This makes it crucial in space and defence applications used in nuclear technologies.
What Are the Health Risks of Exposure to Beryllium?
Despite its benefits, beryllium and beryllium compounds pose serious health risks. Exposure to beryllium dust or fumes can result in acute beryllium disease or chronic beryllium disease—both of which affect the lungs and are linked to long-term adverse health effects.
Airborne beryllium and beryllium particles are especially hazardous in manufacturing and beryllium production facilities. Workers exposed to beryllium can develop sensitisation, leading to immune responses and lung damage. The toxicity of beryllium highlights the need for stringent safety measures.
How Does OSHA Regulate Beryllium Exposure?
The Occupational Safety and Health Administration (OSHA) has implemented strict regulations to protect workers from effects from exposure to beryllium. These rules include exposure limits, personal protective equipment, and medical surveillance programs.
Occupational safety and health standards require employers to monitor beryllium concentration in the air and ensure that workers are not exposed to beryllium beyond safe thresholds. Proper handling of beryllium powder and containment of beryllium dust are key components of compliance.
Why Is Beryllium So Valuable in Aerospace and Defence?
Due to its thermal stability, light weight, and resistance to fatigue, beryllium is a core material in aerospace and defence systems. Beryllium products are found in missile guidance systems, satellite structures, and high-speed aircraft components.
The major application of beryllium in aerospace reflects its ability to perform in harsh environments. Beryllium is found in parts where low weight and high strength are essential, particularly when paired in beryllium alloys that improve resilience.
What Should You Know About the Future of Beryllium Use?
As industries move toward more advanced technologies, the applications of beryllium are expected to grow. From telecommunications to green energy, the demand for this light metal and its compounds will likely continue to rise.
However, sustainability and safety are paramount. Responsible mining of sources of beryllium and strict adherence to health administration standards will be crucial to balancing its benefits with the occupational safety and health of workers handling this powerful element.
Key Takeaways
-
Beryllium is a lightweight, strong metal with atomic number 4, crucial to aerospace, medical, and nuclear industries.
-
The main ores of beryllium are beryl and bertrandite; beryllium was developed industrially through chemical extraction.
-
Beryllium is used in high-performance alloy applications, x-ray technology, and neutron moderation.
-
Its chemical properties include resistance to corrosion, high thermal performance, and low atomic number.
-
Exposure to beryllium can cause severe adverse health effects, necessitating strict occupational safety and health regulations by OSHA.
-
Beryllium and its compounds are monitored to reduce risks in manufacturing environments.
-
The future of beryllium lies in innovation, balanced with sustainability and worker safety.
-
Understanding the periodic table and beryllium’s place in it helps contextualise its broader scientific importance.
-
Protective measures for workers exposed to beryllium include controlling beryllium dust, monitoring beryllium concentration, and preventing airborne beryllium.
-
Continued research and regulation will shape the safe advancement of beryllium technologies.
Discovery of Beryllium
In 1798 French chemist René Haüy noticed similarities in the crystal structures and properties of beryl and emerald despite their differences in colour, a fact that had been noted by the ancient Egyptians. But René Haüy deducted that this was due to them both containing the same as yet unknown element and approached a specialist in chemical analysis Nicolas Louis Vauquelin. At the time it was common practise to taste substances as part of the analysis and Vauquelin tasted the substance which was sweet, and unknown to him toxic, so Vauquelin named the substance ‘glyceynum’ from the Greek word ‘glykis’ meaning sweet.
Beryllium was isolated separately by French chemist Antoine Alexandre Bussy and German physician and chemist Friederich WÖhler in 1828. They both reacted beryllium chloride and potassium in a platinum crucible and yielded beryllium and potassium chloride. Friederich Wöhler renamed the element ‘beryllos’ from the mineral beryl however Bussy preferred the name ‘glucinium’ and it wasn’t until 1957 that it was officially called beryllium.
Sources
Beryllium occurs naturally and can be found in bertrandite and Beryl ore. The United States, China and Kazakhstan are the only countries that commercially process the ore and produce it from exposing the ore to sulphuric acid to produce a sulphate and refined through a chemical process which produce Beryllium Hydroxide.
Uses
Beryllium is a strong lightweight metal which is non magnetic, can absorb immense amounts of heat and resists structural change in extremely cold temperatures.

Beryllium is used by NASA to make mirrors for the Webb Telescope. The telescope is to be sent into outer space requiring a substance that will keep its shape in the cold temperatures of space about -228°c or -379°f. The mirrors in the image on the left have just been tested at NASA’s X-ray & Cryogenic Facility to ensure they maintain their shape at -248°c or -415°f.
Beryllium can withstand very high temperatures with a melting point of 1287 °C it is often used in rockets, missiles and satellites.
The materials high heat capacity also makes it an ideal for heat sinks in electronic devices.
Additionally it is also transparent to X-rays and so is used as windows for x-ray equipment.
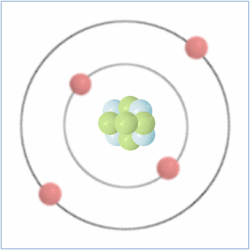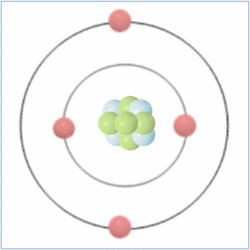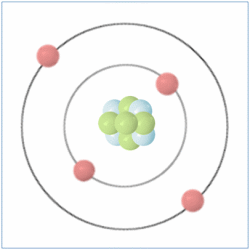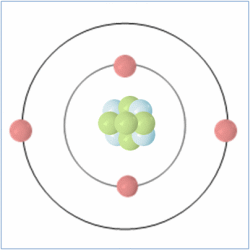
Shell Structure
Protons = 3
Neutrons =4
Electrons=3
|
s |
p |
d |
f |
|
|
1 |
2 |
|||
|
2 |
2 |
|||
|
3 |
||||
|
4 |
||||
|
5 |
||||
|
6 |
||||
|
7 |
Absorption Lines
Emission Lines