Radioactive Decay is the spontaneous change to the nucleus of an unstable atom.
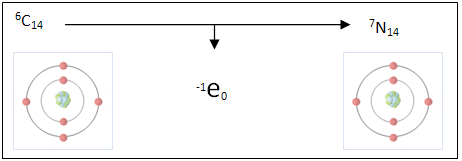
Elements are arranged in the periodic table by their atomic number which is the number of protons in its nucleus. Although every atom of an element has the same number of protons, some elements have varying amounts of neutrons. For example carbon is most commonly found in the form of 6C12 meaning that it has 6 protons and 6 neutrons in its nucleus (neutrons = nucleons – protons), however another form of carbon found naturally is 6C14 meaning that although it still has the 6 protons it also has an extra pair of neutrons. This form of carbon containing the extra pair is known as an isotope or nuclide of carbon (carbon-14).
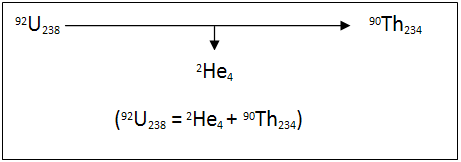
Alpha decay is when the nucleus of an unstable isotope spontaneously changes into another nucleus, producing an alpha particle in the process. An alpha particle is a particle made up of two protons and two neutrons like a helium atom. An example of this process is uranium-238 which forms a thorium-234 and an alpha particle.
Radioactive decay occurs when an isotope of an element like carbon-14 which is unstable and spontaneously changes. In the case of carbon-14 converts one of its extra neutrons into a proton and electron forming a nitrogen ion (an ion is an atom that has either gained or lost an electron giving it positive or negative electrical charge). This reaction takes place in the form of beta decay which requires the loss or gain (in this case loss) of an electron.