Explore the World Through Geography, Natural Resources & Daily History
Clear, reliable and engaging guides that help you understand our planet — from UK geography education to global natural resources and On This Day history events.
Explore, discover, and learn about the wonders of our world! At Earth Site, we’re passionate about bringing geography, history, and science to life for curious minds of all ages. Whether you’re delving into historical events, uncovering the mysteries of the natural world, or seeking interactive resources, you’re in the right place.
Here, you can uncover the stories behind historical events, explore the natural wonders of our planet, and gain valuable insights into how the Earth’s systems shape our daily lives. From the towering peaks of mountain ranges to the far-reaching impacts of human innovation, we aim to make every topic both engaging and informative.
Start your journey of discovery with us today, and let’s make learning an adventure!
What We Cover
Earth Site brings together engaging and accessible educational content designed to help you understand the world, its history, and its natural systems.
🌍 Geography Education (UK & Worldwide)
We publish clear, easy-to-understand geography resources for students, teachers and curious learners. Our guides support geography education in the UK and cover physical geography, climate, ecosystems, population, and global development.
⛏️ Natural Resources & Environmental Geography
Explore detailed country profiles covering natural resources, mining, energy, geology and global environmental challenges. We show how nations manage minerals, water, land and ecosystems, and why these resources matter.
📅 On This Day in History
Every day has a story. Our On This Day history series features major events, anniversaries, traditions, and cultural milestones from around the world — with timelines, context, and fun facts.
TIMELINE
Improper Fractions Test
Your Improper Fractions Test may take a minute to load. If you have to navigate away from this page for any reason, don’t worry, you will have the option to resume your test from the point you left upon your return. The test may have multiple choice, multiple answer or true/false questions and is timed but has no time limit. It should be considered an aid to study for exams or merely a test of your knowledge base giving you indication of areas you may need further study in. Once you have completed your test you will be able to review your results. This will give you some indication of the area you need further study or which areas you are proficient. We recommend you take the Improper Fractions Test before reading the subject matter. If you score 80% or more than you have the option of skipping the section but if you score less than 80% we recommend you read the material associated. Then try the test again until you are able to gain a passing result of 80% correct. Error: Embedded data could not be displayed. Why Use the Improper Fractions Test? As previously stated tests give the user indication of their strengths and weaknesses. This allows them to spend less time studying things they are proficient in and spend more time improving their knowledge where they may have gaps. Tests are also a great tool for revision because they require the user to recall information they may have previously learnt, be it recently or some...
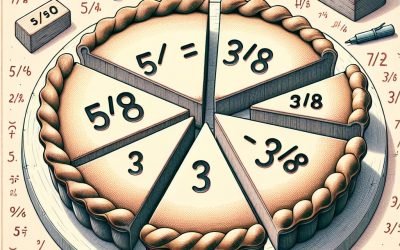
Simplifying or Reducing Fractions Test
Your Simplifying Fractions Test may take a minute to load. If you have to navigate away from this page for any reason, don’t worry, you will have the option to resume your test from the point you left upon your return. The test may have multiple choice, multiple answer or true/false questions and is timed but has no time limit. It should be considered an aid to study for exams or merely a test of your knowledge base giving you indication of areas you may need further study in. Once you have completed your test you will be able to review your results. This will give you some indication of the area you need further study or which areas you are proficient. We recommend you take the Simplifying Fractions Test before reading the subject matter. If you score 80% or more than you have the option of skipping the section but if you score less than 80% we recommend you read the material associated. Then try the test again until you are able to gain a passing result of 80% correct. Error: Embedded data could not be displayed. Why Use the Simplifying Fractions Test? As previously stated tests give the user indication of their strengths and weaknesses. This allows them to spend less time studying things they are proficient in and spend more time improving their knowledge where they may have gaps. Tests are also a great tool for revision because they require the user to recall information they may have previously learnt, be it recently or some...
Equivalent Fractions Test
Your Equivalent Fractions Test may take a minute to load. If you have to navigate away from this page for any reason, don’t worry, you will have the option to resume your test from the point you left upon your return. The test may have multiple choice, multiple answer or true/false questions and is timed but has no time limit. It should be considered an aid to study for exams or merely a test of your knowledge base giving you indication of areas you may need further study in. Once you have completed your test you will be able to review your results. This will give you some indication of the area you need further study or which areas you are proficient. We recommend you take the Equivalent Fractions Test before reading the subject matter. If you score 80% or more than you have the option of skipping the section but if you score less than 80% we recommend you read the material associated. Then try the test again until you are able to gain a passing result of 80% correct. Error: Embedded data could not be displayed. Why Use the Equivalent Fractions Test? As previously stated tests give the user indication of their strengths and weaknesses. This allows them to spend less time studying things they are proficient in and spend more time improving their knowledge where they may have gaps. Tests are also a great tool for revision because they require the user to recall information they may have previously learnt, be it recently or some...
Calculating Fractions Test
Your Calculating Fractions Test may take a minute to load. If you have to navigate away from this page for any reason, don’t worry, you will have the option to resume your test from the point you left upon your return. The test may have multiple choice, multiple answer or true/false questions and is timed but has no time limit. It should be considered an aid to study for exams or merely a test of your knowledge base giving you indication of areas you may need further study in. Once you have completed your test you will be able to review your results. This will give you some indication of the area you need further study or which areas you are proficient. We recommend you take the Calculating Fractions Test before reading the subject matter. If you score 80% or more than you have the option of skipping the section but if you score less than 80% we recommend you read the material associated. Then try the test again until you are able to gain a passing result of 80% correct. Error: Embedded data could not be displayed. Why Use the Calculating Fractions Test? As previously stated tests give the user indication of their strengths and weaknesses. This allows them to spend less time studying things they are proficient in and spend more time improving their knowledge where they may have gaps. Tests are also a great tool for revision because they require the user to recall information they may have previously learnt, be it recently or some...
Order of Functions Test
Your Order of Functions Test may take a minute to load. If you have to navigate away from this page for any reason, don’t worry, you will have the option to resume your test from the point you left upon your return. The test is multiple choice and not timed. It should be considered an aid to study for exams or merely a test of your knowledge base giving you indication of areas you may need further study in. Once you have completed your test you will be able to review your results. This will give you some indication of the area you need further study or which areas you are proficient. We recommend you take the Order of Functions Test before reading the subject matter. If you score 80% or more than you have the option of skipping the section but if you score less than 80% we recommend you read the material associated. Then try the test again until you are able to gain a passing result of 80% correct. Error: Embedded data could not be displayed. Why Use the Order of functions test? As previously stated tests give the user indication of their strengths and weaknesses. This allows them to spend less time studying things they are proficient in and spend more time improving their knowledge where they may have gaps. Tests are also a great tool for revision because they require the user to recall information they may have previously learnt, be it recently or some time ago. This recall of information improves its...
Oxygen
Oxygen is the third most abundant element in the universe and highly reactive forming compounds with almost all the other elements Basic Information Discovery Sources The Ozone Layer Uses of Oxygen Living Organisms Oxygen rich combustion Fuel Cells Cell Structure Absorption Lines Emission Lines Oxygen Basic Information Classification: non-metal Atomic Mass: 15.99 u Density: 1.429 g/l Colour: None Boiling Point: 90.188 K (-182.962°C) Melting Point: 54.36 K (-218.79°C) Discovery Oxygen was discovered in 1774 by English chemist Joseph Priestly, which he isolated by heating mercuric oxide (HgO). Sources Oxygen is the third most abundant element in the universe and highly reactive forming compounds with almost all the other elements. At the creation of earth it combined with silicon to create our crust, hydrogen to create water and it makes up 20.8 % of the air in atmosphere. The Ozone Layer Ozone is an allotrope (an allotrope is another form of an element for example graphite and diamond are allotropes of carbon) of oxygen with the chemical symbol O3. In our upper atmosphere the ozone layer protects the inhabitants of earth from harmful U.V. rays released by the sun. Ozone layer is also found much closer to the earth’s surface where its effects can cause respiratory problems for people especially those who suffer from asthma etc. Uses Living Organisms Oxygen is necessary for all life. It is used by every cell in living organisms, except somatic cells (sperm and egg cells), to produce the energy they require through aerobic...
Our Moon
Shortly after the formation of our moon by the Great Impact event our neighbour would have been much closer to Earth Our Moon The Great Impact Hypothesis Tidal Forces Craters on the Moon Solar Eclipse Total Eclipse Partial Eclipse Luna Eclipse Quick Stats Our Moon The moon has been a welcomed companion in the sky providing light for human and animal kind alike since life began. It has been studied for centuries with maps of its surface being created by many including Galileo. Shortly after the moon’s formation by the Great Impact event (see below), our neighbour would have been much closer to Earth. At only 20 or 30 thousand kilometres away the moon’s tidal effects would have been greatly increased and although water wouldn’t have been present in liquid form, the liquid magma may have been affected. If this was the case then the tidal effects on the magma would have kept the temperature of the Earth’s crust hotter for longer and may have affected how our planet’s surface formed. The Great Impact Hypothesis In 1975 two papers were published with a new theory that would change our picture of how Earth was formed and its relationship with our moon. One paper by William K. Hartmann and Donald Davis and another by Alfred G. W. Cameron and William Ward were written to explain why the moon has such a small metallic core which seems to be abnormal to the other celestial bodies in our solar system. The theory is called ‘The Giant Impact Hypothesis’ and is now regarded as the most...
Order of Functions
To ensure uniformity, and prevent mistakes, there are rules on the order to calculate maths problems and this is known as the order of functions. What is the order of functions? Brackets in sums Multiplication or Division Addition and Subtraction What is the order of functions? To ensure uniformity, and prevent mistakes, there are rules on the order to calculate maths problems and this is known as the order of functions. In life you may come across a maths problem which looks something like this. Example 1 12 + 4 X 5 This sum can be calculated in two ways. Either you add the 12 to the 4 equalling 16 and then multiply that by 5 giving the answer of 80. Or you may multiply 4 X 5 which is 20 and then add the 12 giving the answer of 32. So as you can see getting the correct order of functions is vital to getting the right answer, but which of these is correct? You may think the logical move would be to calculate as it is written i.e. left to right but this is not the correct order of functions. Brackets in sums The rules for the order of functions are as follows. The first thing to look for in an equation is brackets. If part of the equation is in brackets then this needs to be calculated first. Example 2 5 X (3 + 7) So first you add the 3 and 7 which is 10 and then...
Nuclear Reactions
Nuclear reactions are reactions that change the nucleus of atoms and are caused by special circumstances, such as Nuclear Fusion or Nuclear Fission. What are Nuclear Reactions? Nuclear Fusion Nuclear Fission Forces that Hold Atoms Together How nuclear reactions produce energy What are Nuclear Reactions? Nuclear reactions are reactions that change the nucleus of atoms and are caused by special circumstances, such as Nuclear Fusion or Nuclear Fission. Although Radioactive Decay is a reaction affecting the nucleus of atoms it is spontaneous and would happen naturally due to the instability of atoms requiring no special circumstances or intervention. Nuclear Fusion Nuclear fusion currently only occurs in the unique setting of stars. It is the fusion of atomic nuclei that produces the energy that powers the star (and therefore life on earth). Although it does occur naturally, extreme temperature and pressure are required to begin the process of nuclear fusion. Under the immense gravity of the star the state of the hydrogen changes from gas to plasma and at about 10 million degrees centigrade the process of nuclear fusion can begin. Individual protons combine, with some converting to neutrons, making deuterium, helium and more massive elements. With each fusion of nuclei into more massive elements mass is lost due to binding energy and this loss of mass becomes energy which helps sustain the process and power the star. The energy produced from nuclear fusion is four times more powerful than that produced in nuclear fission and about 4 million times greater than any chemical reaction. Fusion also...
Radioactive Decay
Radioactive Decay is the spontaneous change to the nucleus of an unstable atom. Radioactive decay Alpha Decay Beta Decay Radioactive decay Elements are arranged in the periodic table by their atomic number which is the number of protons in its nucleus. Although every atom of an element has the same number of protons, some elements have varying amounts of neutrons. For example carbon is most commonly found in the form of 6C12 meaning that it has 6 protons and 6 neutrons in its nucleus (neutrons = nucleons – protons), however another form of carbon found naturally is 6C14 meaning that although it still has the 6 protons it also has an extra pair of neutrons. This form of carbon containing the extra pair is known as an isotope or nuclide of carbon (carbon-14). Alpha Decay Alpha decay is when the nucleus of an unstable isotope spontaneously changes into another nucleus, producing an alpha particle in the process. An alpha particle is a particle made up of two protons and two neutrons like a helium atom. An example of this process is uranium-238 which forms a thorium-234 and an alpha particle. Beta Decay Radioactive decay occurs when an isotope of an element like carbon-14 which is unstable and spontaneously changes. In the case of carbon-14 converts one of its extra neutrons into a proton and electron forming a nitrogen ion (an ion is an atom that has either gained or lost an electron giving it positive or negative electrical charge). This reaction takes place in the form of beta decay which requires the loss or gain...
Nuclear Fission
Nuclear fission occurs when a large atomic nucleus is split by means of neutron bobardment into smaller nuclei. Nuclear Fission Where the energy comes from in Nuclear Fission? Induced Nuclear Fission Nuclear Fission Reactors Why use Uranium for Nuclear Fission? Difficulties with nuclear fission as an energy source Nuclear Fission and the environment Plutonium Fast Breeder Reactors Nuclear Fission Nuclear fission occurs when an atomic nucleus is split into smaller nuclei. Unlike Radioactive decay, which is spontaneous and not considered a nuclear reaction, nuclear fission must be initiated by neutrons. Where the energy comes from in Nuclear Fission? With the exception of hydrogen –which only contains a proton in its nucleus – all the elements are less massive than the mass of their constituent parts. For example helium contains four nucleon, 2 protons and 2 neutrons, in its nucleus. If you had added the mass of the individual nucleons together they would have more mass than a helium nucleus. So where has the mass gone? The missing mass has become the energy required to hold the nucleus together (the strong nuclear force) and this energy is known as the binding energy. The table above shows the amount of binding energy in each nucleon of each element. The higher binding energy per nucleon the more stable the nucleus of the element is and all elements want to be as stable as possible. As you can see from the table Iron is the most stable (has the most binding energy per nucleon) element and as such neither nuclear fusion nor fission is...
Binding Energy
Binding Energy is the energy required to break up the bonds of atoms or the energy released when small atoms combine. Binding Energy Nuclear Binding Energy Calculating Binding Energy Calculating the Assumed Mass Binding Energy per Nucleon Binding Energy This is the energy required to break up the bonds of atoms or the energy released when small atoms combine. There are two types of binding energy including electron binding energy (the energy required to overcome the electromagnetic force holding the electrons in orbit) and the Nuclear Binding Energy. Nuclear Binding Energy This can be thought of as either; the energy required to break up the bonds of the nucleus of an atom or the energy released when nucleons combine. The table above shows the amount of binding energy in each nucleon of each element. The higher binding energy per nucleon the more stable the nucleus of the element is and all elements want to be as stable as possible. As you can see from the table Iron is the most stable (has the most b-energy per nucleon) element and as such neither nuclear fusion nor fission is possible. As you can see from the table, all elements with less mass then iron will release energy through nuclear fusion as these element gain nuclear b-energy per nucleon as their mass increases. Elements with more mass than iron have less b-energy per nucleon and so they release energy from splitting into less massive elements through nuclear fission. Calculating Binding Energy A nucleus – except in the case of hydrogen...