Explore the World Through Geography, Natural Resources & Daily History
Clear, reliable and engaging guides that help you understand our planet — from UK geography education to global natural resources and On This Day history events.
Explore, discover, and learn about the wonders of our world! At Earth Site, we’re passionate about bringing geography, history, and science to life for curious minds of all ages. Whether you’re delving into historical events, uncovering the mysteries of the natural world, or seeking interactive resources, you’re in the right place.
Here, you can uncover the stories behind historical events, explore the natural wonders of our planet, and gain valuable insights into how the Earth’s systems shape our daily lives. From the towering peaks of mountain ranges to the far-reaching impacts of human innovation, we aim to make every topic both engaging and informative.
Start your journey of discovery with us today, and let’s make learning an adventure!
What We Cover
Earth Site brings together engaging and accessible educational content designed to help you understand the world, its history, and its natural systems.
🌍 Geography Education (UK & Worldwide)
We publish clear, easy-to-understand geography resources for students, teachers and curious learners. Our guides support geography education in the UK and cover physical geography, climate, ecosystems, population, and global development.
⛏️ Natural Resources & Environmental Geography
Explore detailed country profiles covering natural resources, mining, energy, geology and global environmental challenges. We show how nations manage minerals, water, land and ecosystems, and why these resources matter.
📅 On This Day in History
Every day has a story. Our On This Day history series features major events, anniversaries, traditions, and cultural milestones from around the world — with timelines, context, and fun facts.
TIMELINE
Nuclear Fusion
Nuclear Fusion is the name given to the the event when the nuclei of two atoms combine creating a new heavier atomic nucleus. What is Nuclear Fusion? Overcoming the Electromagnetic Force Nuclear Fusion in Stars Energy Production through Nuclear Fusion What is Nuclear Fusion? Nuclear Fusion is a phenomenon where by the nucleus of two atoms fuse together creating a new heavier atomic nucleus. When two relatively small atomic nuclei have very high energies they are able to bond or fuse together making larger atoms. The atomic nuclei require a lot of energy to overcome the effects of the electromagnetic force before it is possible for nuclear fusion to occur. Overcoming the Electromagnetic Force Normally the electromagnetic force causes atomic nuclei to repel each other due to their positive charge, much like the same poles on a magnet. Electromagnetic force which causes the repulsive action quadruples as the distance between the two protons halves. This means that it takes a lot of energy to get these two protons, and two nuclei together. If they are able to get close enough another fundamental force, The Strong Force, comes into play and this force is much stronger than the electromagnetic force. It is the force that holds all nucleons together but they must be within one femtometres (the nucleus of an average atom is 4 femtometres in diameter). A = The Nucleus of the atom contains the protons and neutrons. Despite accounting for the majority of an atom’s mass, the nucleus procures a minute proportion of the total space. The Diameter of the nucleus is approximately 4 femtometres or 4...
Nitrogen Cycle
The Nitrogen cycle refers to the process that recycles nitrogen on earth ensuring this important commodity remains abundant. What is the Nitrogen Cycle? Nitrogen Cycle in Plants Nitrogen Cycle in Animals Nitrogen Cycle in Aquatic Life What is the Nitrogen Cycle? The Nitrogen cycle refers to the process that recycles nitrogen on earth ensuring this important commodity remains abundant. For healthy growth, plants need a good supply of nutrients and the most important of these is nitrogen. If these nutrients are depleted from the soil plant life is unable to thrive so maintaining a healthy nutrient rich soil is very important for both the plants and animals that depend on them. Nature can continue to thrive via recycling these valuable nutrients through various processes. Nitrogen is recycled throughout life forms in a process known as the Nitrogen Cycle. (Environmental Protection Agency All EPA images are in the public domain) Nitrogen Cycle in Plants Plants are unable to take up nitrogen in its basic form but instead they can use compounds of nitrogen such as nitrates and ammonia which are general produced by microorganisms in the soil and around the roots of legumes. The microorganism use an enzyme called Nitrogenase to convert the nitrogen into ammonia. The enzyme does not work with oxygen and so these bacteria have adapted the parts of the cells required for nitrogen fixing (conversion of nitrogen into nitrogen compounds) to ensure anaerobic conditions exist. Nitrogen Cycle in Animals Plants take up nitrate ions from the soil through their roots and use these nitrates...
Nitrogen
Nitrogen is the most abundant gas in our atmosphere making up approximately 78% with 21% oxygen, 0.93% argon, and rest is made up other various gases. Basic Information Discovery of Nitrogen Sources of Nitrogen Uses of Nitrogen Liquid Nitrogen The Nitrogen Cycle Atmospheric Nitrogen Causing Aurora Nitrogen’s Cell Structure Absorption Lines of Nitrogen Emission Lines of Nitrogen Nitrogen (from the Latin words “nitron” meaning native soda and “gene” meaning from) Classification: Non-Metallic Atomic Mass: 14.0067 g/mol Density: 1.251g/l Colour: No Colour Boiling Point: 77.36K (-195.79°C) Melting Point: 63.05K (-210.1°C) Critical Temperature: 126.2K (-146.9°C) Discovery of Nitrogen Discovered by Scottish Physician Daniel Rutherford in 1772. Rutherford isolated nitrogen when he was studying carbon dioxide. Sources Nitrogen is the most abundant gas in our atmosphere making up approximately 78% with 21% oxygen, 0.93% argon, 0.039% carbon dioxide and 0.031% makes up other various gases. Nitrogen is obtained from the fractional distillation of liquefied air. This process requires the air to be cooled to -200°c so it becomes a liquid. The Liquefied air is then pumped into a chamber where the temperature increases by several degrease. When the air reaches -190°c the nitrogen in the air boils and becomes a gas once more separating into the top chamber. The remaining liquid is a mixture of Liquid oxygen with a small amount of liquid argon. These two are then separated in a second chamber through the same process. Uses Liquid Nitrogen is nitrogen gas that has been cooled sufficiently enough for it to revert to...
Metis – Moon of Jupiter
Metis is one of Jupiter’s many moons, which is currently believed to have at least 63. It is one of four moons that make up the ‘Inner Group’. Where in the Solar System? The Inner Group Metis Where in the Solar System? Metis is one of Jupiter’s many moons, which is currently believed to have at least 63. It is one of four moons that make up the ‘Inner Group’. The Inner Group This is a group of four moons and as the name suggest they are situated closest to Jupiter at distances ranging from 128,000 – 200,000 km and taking between 7 and 16 hours to orbit the host planet. The Inner Group consists of Metis, Adrastea, Amalthea and Thebe, all of which are ‘irregular moon’ meaning they are not spherical due to their mass being too low (when cosmic bodies mass are large enough their gravity is able to shape the object into a sphere). Metis Metis is Jupiter’s closest moon, orbiting at around 128,000 km from the planet’s surface which is less than a third the distance of our own moon and earth (distance between earth and its moon is 384,400km). Metis got its name from the Greek mythical goddess of the same name, who was the first spouse of Zeus. It was discovered on the 4th of March 1979 by Stephen P Synnott, an American Astronomer and scientist working on NASA’s Voyager mission. Metis hurtles through space at an impressive 31.501 km/s (average speed) which is 70,465 miles per hour and takes...
Meteorology – Understanding Weather
Meteorology is the study of weather; its patterns, causes and effects. The Study of meteorology has enabled us to predict the weather with remarkable accuracy. Temperature variations Airflow and Winds Rain The Water Cycle Evaporation Sublimation Transpiration Condensation Clouds Temperature variations Based on data from the World Meteorological Organization temperatures on dry land range from -89.2˚C (-128.6˚F), recorded at Vostok Station in Antarctica, to 56.7˚C (136.4˚F), recorded at Death Valley in the US. Temperatures in the ocean vary from around 36˚C (96.8˚F) in the Persian Gulf to -2˚C (28.5˚F) Antarctic waters (water on the deep ocean is between 0 to 3˚C). This means that temperatures on land vary by approximately 68˚C (with an average of 16˚C) while oceans vary 38˚C (with an average of 17˚C). Airflow and Winds Temperature variations are the main source of the winds that drive the movement of rain clouds and create hurricanes. As the hot and cold air attempt to equalise it moves and this creates the winds. When these temperature variations are extreme the hot and cold air encircle each other creating a cyclone When atoms or molecules are supplied with heat energy they move around and the more energy supplied the more they move. This movement causes the molecules to take up more space than their colder counterparts. The illustration above shows the relationship between the increase in temperature (energy) and the motion of the air molecules. The molecules do not move in such a uniform pattern but as shown they do take up more space when supplied with heat energy....
Martian Moons – The Two Moons of Mars Phobos and Deimos
Martian Moons – The Two Moons of Mars Phobos and Deimos In the August of 1877 an American astronomer was looking at Mars in hopes of discovering a moon. He instead discovered two Martian Moons; Deimos and Phobos Phobos and Deimos—the mysterious moons of Mars—have intrigued scientists for generations. These tiny satellites orbiting the planet Mars offer clues to the planet’s past and its potential future in human space exploration. In this article, we dive deep into the Martian satellites Phobos and Deimos, unravel fascinating facts about Phobos, and learn how NASA science continues to probe their origins and significance. Whether you’re a space enthusiast, a student of astronomy, or just curious about what orbits the surface of Mars, this article will offer a thorough overview of the Mars’ moons, their orbits, potential missions, and why they’re crucial for future Mars exploration. Article Outline What Are the Moons of Mars and Why Are They Important? How Were Phobos and Deimos Discovered? What Are the Main Differences Between Phobos and Deimos? How Do the Martian Moons Orbit Mars? Could Phobos Crash Into Mars? What Has NASA Learned from Mars Missions? What Is the Origin of the Martian Moons? What Are the Plans for Future Moon Exploration? How Do Phobos and Deimos Compare to Earth’s Moon? Why Is Phobos Key to a Mars Sample Return Mission? Naming the Moons of Mars Shape and Orbit of the Martian Moons Origin of the Martian Moons Phobos Deimos Quick Comparison of Martian Moons What Are the Moons of Mars and Why Are They Important? Mars has two small moons, Phobos and Deimos, which are...
Mammals
The term Mammals is the name given to a group of animals that produce milk for their young (in mammary glands) which currently accounts for 5,000 species. What are Mammals? Rise of mammals Common characteristics Hair Three Middle Ear Bones Milk Production from mammary glands Orders of Mammals What are Mammals? The term Mammals is the name given to a class of animals that produce milk for their young (in mammary glands) which currently accounts for 5,000 species. They have populated every part of the globe, from whales and dolphins in the sea, bats in the sky, moles that live underground and land mammals that live on every continent. Rise of mammals Although mammals existed at the time of the dinosaurs their population and diversity exploded during the Paleocene epoch – a 10 million year period that immediately followed the dino-extinction event. For around 160 million years the mammals lived in the shadow of dinosaurs but some 65.5 million years ago the Cretaceous-Tertiary extinction event (also known as the K-T event) occurred, wiping out the dinosaurs and dawning the Age of Mammals. Common characteristics As well as producing milk for their young mammals have other equally important similarities or characteristics. These include the production of hair and the three middle ear bones. Hair All mammals have it and any animal with it is a mammal1. Even aquatic mammals such as dolphins, whales and porpoises have hair somewhere on the body of adults. This hair is used by mammals for many purposes such...
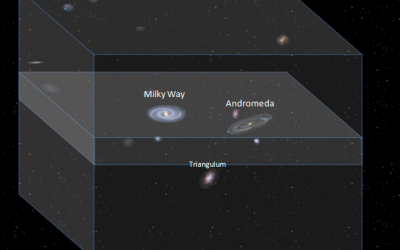
Local Galactic Group
The Local galactic group is the term we use for a group of over thirty galaxies in our local vicinity. What is our Local Galactic Group? Which Galaxies makeup our Local Galactic Group? Milky Way Group Andromeda The Triangulum spiral Galaxy (M33) Galaxies in our Local Group – Quick Reference What is our Local Galactic Group? Galaxies in a super cluster are not spread evenly but seem to be clumped together due to gravity into groups. The Local group is the term we use for a group of over thirty galaxies in our local vicinity (although various sources suggest more member galaxies these are not confirmed) and our group was first recognised by American astronomer and physicist Edwin Hubble. Which Galaxies makeup our Local Galactic Group? The two main Galaxies within our group are the Milky Way galaxy and the Andromeda galaxy (M31), each with their own surrounding group of smaller galaxies drawn in by the massive gravitational pull of these giants. Evidence suggests that these two very large galaxies were formed from absorption and culminations of smaller galaxies1. Observations of our two galaxies also suggest that they are being drawn closer together at a speed of 300km (or 180mi) a second and one day, in about several billion years from now, they too will merge as one super galaxy. This process will not happen quickly and it will take over about 2 billion years2 for the unity to be complete. Another smaller but still very large galaxy (third largest of the group but about...
Liquid Nitrogen
Liquid Nitrogen is nitrogen gas (its normal state on earth) that has been cooled sufficiently enough for it to change into its liquid state. What is liquid Nitrogen? Where Does Liquid Nitrogen Come From? Use of Liquid Nitrogen Use of Liquid Nitrogen in Science Medical Applications Use in Special effects Liquid Nitrogen use in Cuisine Safety Concerns with Liquid Nitrogen What is liquid Nitrogen? Liquid Nitrogen is nitrogen gas (its normal state on earth) that has been cooled sufficiently enough for it to change into its liquid state. Nitrogen becomes a liquid (at normal atmospheric pressure) between -210o C to -196o C (63 K to 77.2 K or -346°F to -320.44°F). Where Does Liquid Nitrogen Come From? Nitrogen is obtained industrially from fractional distillation, a process that involves cooling air down to -200° C so it becomes a liquid. This is then allowed to heat in a chamber by several degrees until the air becomes -190 C and the nitrogen becomes a gas once more. At this point the collected nitrogen can then be cooled to -195 ˚C or more when it becomes liquid Nitrogen (nitrogen’s boiling point – when it becomes a gas – is -195.8 ˚C). Air is made up of 78% nitrogen, 21% oxygen and 1% of other substances. This means that air is a very good source of nitrogen and the most efficient way of extracting it is through fractional distillation. In many cases fractional distillation required heating the original substance to extract its required parts but fractional distillation of air requires cooling to low temperatures. As the nitrogen is already...
Lithium – alkali metal
Lithium is a soft silver-white metal with a range of applications including medication,batteries and fireworks. Basic Information Discovery of Lithium Sources of Lithium Uses of Lithium Lithium Tablets Lithium Batteries Lithium In Fireworks Lithium’s Cell Structure Absorption Lines of Lithium Emission Lines of Lithium Lithium (from the Greek Lithos meaning Stone) Classification: Alkali metal Atomic Mass: [6.941 (2)] g/mol Density: 0.534g/cm3 Colour: metallic silver or greyish white Boiling Point: 1615K (1342°C) Melting Point: 453.69K (180.54°C) Critical Temperature:3223K (2950°C) Discovery of Lithium Lithium (3Li) was first discovered in the mineral petalite (LiAlSi4O10) by Johann Arfvedson in Sweden 1817. Sources It is harvested from compounds found in most igneous rocks, clay, brine sources and through electrolysis of compounds like lithium chloride. 3Li is a highly reactive and flammable metal like all the alkali metals and is only found in nature as part of a compound. A pure sample is a metallic silver metal however this very quickly changes into a dull whitish grey or black colour as it reacts with moisture or oxygen in the air. 3Li is the only alkali metal that reacts with nitrogen. To prevent reactions with other elements it is usually stored in mineral oil which acts as a barrier or protective seal. Uses In medicine 3Li is used to regulate the sodium levels in the muscles and nerves reducing the effects of mania in those that suffer from manic depression or manic episodes. 3Li helps reduce some of the symptoms associated with the illness such as hyperactivity, insomnia, aggression...
Hydrogen
Hydrogen is the lightest and most abundant element in the universe. Basic Information Discovery of Hydrogen Sources of Hydrogen Uses of Hydrogen The Hydrogen Bomb Airships Hydrogen Fuel Cells Hydrogen’s Cell Structure Absorption Lines of Hydrogen Emission Lines of Hydrogen Hydrogen (from the Greek hudor (meaning water) and gennan (meaning generate) Classification: Non-metallic Atomic Mass: 1.00794 g/mol Density: 0.08988g/cm3 Colour: None Boiling Point: 20.268K (-252.87°C Melting Point: 14.01K (-259.14°C) Critical Temperature: 33K (-240°C) Discovery Hydrogen was discovered in 1766 by English physicist Henry Cavendish. Cavendish conducted numerous experiments and eventually identified that hydrogen was a unique gas with its own set of properties. Fast forward to today, and the significance of hydrogen is more apparent than ever. This little molecule holds incredible potential as a clean and renewable energy source. Scientists and researchers worldwide are tirelessly working to harness its power and overcome some of the current challenges that come with its production and storage. Sources Hydrogen is the most abundant element in the universe with nearly 90% of all visible atoms being hydrogen. The first atoms ever created after the Big Bang would have been that of hydrogen and helium which eventually culminated into stars. Due to the intense heat and pressure within the stars the hydrogen is in a state known as plasma and nuclear fission turns the hydrogen atoms into Helium, the next most abundant element. On earth Hydogen is most abundant in the sea where it has been mixed with oxygen to create water. Uses Hydrogen is used in the production of ammonia (NH3), ethanol (alcahol(C2H5OH)) and hydrogen Chloride (HCL) among many other uses. Hydrogen has...
The Lighter Side of Science: Exploring the Wonders of Helium (He)
The Lighter Side of Science: Exploring the Wonders of Helium (He) The Lighter Side of Science: Exploring the Wonders of Helium (He) Basic Information Discovery of Helium Sources of Helium Uses of Helium Liquid Helium in Superconductors Use of Helium by Divers Helium in Balloons and Airships Helium’s Cell Structure Absorption Lines of Helium Emission Lines of Helium Helium (from the Greek helios meaning Sun) Classification: Non-metallic Atomic Mass: 1.00794 g/mol Density: 0.08988g/cm3 Colour: None Boiling Point: 20.268K (-252.87°C Melting Point: 14.01K (-259.14°C) Critical Temperature: 33K (-240°C) The outreaching wave is called the prominence and is composed of ionized Helium that is about 60,000 degrees Kelvin. (Image from NASA) Discovery Discovery: Helium was first detected by French astronomer Pierre Janssen during a total solar eclipse in Guntur, India on the 18th of August 1868 using spectroscopy. It was evident as a bright yellow line (a wavelength of 587.49 nanometres) in the spectrum of the Chromosphere of the Sun but it was assumed that this line was produced by sodium which produces similar yellow lines. But it was On the 20th of October 1868 English astronomer Norman Lockyer observed the same line and concluded that it was produced by an element in the sun that was as yet un-discovered on earth. Lockyer and English chemist Edward Frankland named the element after the Greek word for the Sun (helios). Helium was not isolated on earth until the 26th of March 1895 when a Scottish chemist Sir William Ramsey isolated samples whilst attempting isolate argon. The samples were confirmed to be Helium by Lockyer and English...