🌌 Introduction to Physics
The Science That Explains How the Universe Works
Physics is the science that seeks to understand the fundamental laws of nature—the forces, energies, and interactions that shape everything from the tiniest particles to the vastness of galaxies. It explores how objects move, why they move, and what rules govern their behavior.
At its heart, physics asks questions like:
-
Why do things fall?
-
How does light travel?
-
What causes electricity and magnetism?
-
What is time, space, energy, and matter?
Often called the “foundation of all sciences,” physics underpins everything from chemistry and biology to engineering and astronomy. Whether it’s explaining why the sky is blue, how bridges stay standing, or how smartphones work, physics provides the framework.
From Isaac Newton’s laws of motion to Albert Einstein’s theory of relativity, and from quantum mechanics to black holes, physics challenges us to think big—and small. It combines deep curiosity with mathematical precision to uncover the rules of reality.
Studying physics not only helps us understand the universe—it equips us with problem-solving skills that drive innovation and technological progress.
Physics by Topic
Sun
The Sun’s Rotation Sections of the Sun The Core The Radioactive Zone The Convection Zone The Photosphere The Chromosphere The Corona As illustrated the sun rotates in an anti-clockwise direction as observed from its most northern point. The solid core rotates in much the same way as all the solid planets in the solar system, however as the outer shell is made up from gas and plasma, it spins at different speeds from the equator as it does in the poles (yet still in the same direction). At the equator the sun rotates faster, taking 26.8 Earth days to make a full rotation, whereas it takes up to 36 Earth days to make one rotation at the poles. The Sun’s Rotation The rotation of the suns outer layers have a very powerful effect on the magnetic field produced by the sun. Because the material of the sun rotates at different speeds it causes the magnet lines of force to become tangled and even tear. Magnetic lines of force can be viewed on a normal magnet using iron fillings. By placing a piece of paper above a magnet and sprinkling iron filings on the paper, curved lines appear between the north and south poles. The two ends of torn lines can show up on the Photosphere as sunspots where they cool the plasma considerably. Another effect of this can be solar flares that are massive expulsion of energy when the lines snap and reconnect and occasionally this forces an eruption of actual plasma from the Corona, a phenomenon we call Coronal Mass Ejection...
Radioactive Dating
Radioactive dating is a process whereby a person can calculate the age of an object by measuring its rate of radioactive decay. What is Radioactive Dating? Carbon Dating Geological Dating Isotopes used in Geological Dating Radioactive Dating with Uranium-238 What is Radioactive Dating? Radioactive dating is a process whereby a person can calculate the age of an object by measuring its rate of radioactive decay. This is because the rate of decay is consistent with any particular isotope and is not affected by outside environmental influences. A sample of radioactive material will contain a certain amount of the unstable isotope that has not yet decayed into its stable form. By comparing the amount of unstable and stable isotopes we can calculate the age of some materials. In a particular sample of radioactive material it takes a set amount of time for half of the nuclei to succumb to radioactive decay which is called the radioactive half-life. Carbon Dating All life forms intake carbon during their lives and a small proportion of that carbon is in the form of carbon-14 (due to cosmic rays). Within the body of living organism the percentage of carbon-14 and carbon-12 meet a certain level which is sustained until its death. At this point no more carbon is ingested and the ‘clock’ starts. The levels of carbon-14 (6C14) start to drop as it decays into a nitrogen ion (7N14) and by knowing the rate of decay you can compare the levels carbon-12 and carbon-14 to calculate the approximate age of the organic material. This method however is only suitable...
Isotopes – what are they?
Isotopes are the variations of elements. An element is defined by the amount of its protons while an isotope of that element has different amount of neutrons. What are isotopes? How are isotopes formed? Stable Isotopes Unstable Isotopes (Radioactive Isotopes) Alpha Radiation or Alpha Decay Beta Radiation or Beta Decay Radioactive half-life What are Isotopes? Here is the chemical symbol for helium as you might see it on a periodic table. The number at the top is known as the atomic number and is actually the number of protons that are found in the atom. The number at the bottom is the atomic mass of the element. As electrons are considered to little or no mass the atomic weight is the amount of protons and neutrons normally found in that element. While the atomic number of an element will always remain the same (hydrogen will always have one proton, helium will always have two etc) the atomic mass can change naturally. This happens when more neutrons are added to the element in some way. How are isotopes formed? Taking the most basic element, hydrogen, it has a one proton and one electron as shown in the diagram. In the process of nuclear fusion in the sun these hydrogen atoms are split into individual elements of protons and electrons that float around in the form of plasma. The pressure and heat that cause the hydrogen to turn to plasma, rather than gas, also make it easy for the particles to form new atoms. When one of these free protons collides with a hydrogen atom...
Our Moon
Shortly after the formation of our moon by the Great Impact event our neighbour would have been much closer to Earth Our Moon The Great Impact Hypothesis Tidal Forces Craters on the Moon Solar Eclipse Total Eclipse Partial Eclipse Luna Eclipse Quick Stats Our Moon The moon has been a welcomed companion in the sky providing light for human and animal kind alike since life began. It has been studied for centuries with maps of its surface being created by many including Galileo. Shortly after the moon’s formation by the Great Impact event (see below), our neighbour would have been much closer to Earth. At only 20 or 30 thousand kilometres away the moon’s tidal effects would have been greatly increased and although water wouldn’t have been present in liquid form, the liquid magma may have been affected. If this was the case then the tidal effects on the magma would have kept the temperature of the Earth’s crust hotter for longer and may have affected how our planet’s surface formed. The Great Impact Hypothesis In 1975 two papers were published with a new theory that would change our picture of how Earth was formed and its relationship with our moon. One paper by William K. Hartmann and Donald Davis and another by Alfred G. W. Cameron and William Ward were written to explain why the moon has such a small metallic core which seems to be abnormal to the other celestial bodies in our solar system. The theory is called ‘The Giant Impact Hypothesis’ and is now regarded as the most...
Nuclear Reactions
Nuclear reactions are reactions that change the nucleus of atoms and are caused by special circumstances, such as Nuclear Fusion or Nuclear Fission. What are Nuclear Reactions? Nuclear Fusion Nuclear Fission Forces that Hold Atoms Together How nuclear reactions produce energy What are Nuclear Reactions? Nuclear reactions are reactions that change the nucleus of atoms and are caused by special circumstances, such as Nuclear Fusion or Nuclear Fission. Although Radioactive Decay is a reaction affecting the nucleus of atoms it is spontaneous and would happen naturally due to the instability of atoms requiring no special circumstances or intervention. Nuclear Fusion Nuclear fusion currently only occurs in the unique setting of stars. It is the fusion of atomic nuclei that produces the energy that powers the star (and therefore life on earth). Although it does occur naturally, extreme temperature and pressure are required to begin the process of nuclear fusion. Under the immense gravity of the star the state of the hydrogen changes from gas to plasma and at about 10 million degrees centigrade the process of nuclear fusion can begin. Individual protons combine, with some converting to neutrons, making deuterium, helium and more massive elements. With each fusion of nuclei into more massive elements mass is lost due to binding energy and this loss of mass becomes energy which helps sustain the process and power the star. The energy produced from nuclear fusion is four times more powerful than that produced in nuclear fission and about 4 million times greater than any chemical reaction. Fusion also...
Nuclear Fission
Nuclear fission occurs when a large atomic nucleus is split by means of neutron bobardment into smaller nuclei. Nuclear Fission Where the energy comes from in Nuclear Fission? Induced Nuclear Fission Nuclear Fission Reactors Why use Uranium for Nuclear Fission? Difficulties with nuclear fission as an energy source Nuclear Fission and the environment Plutonium Fast Breeder Reactors Nuclear Fission Nuclear fission occurs when an atomic nucleus is split into smaller nuclei. Unlike Radioactive decay, which is spontaneous and not considered a nuclear reaction, nuclear fission must be initiated by neutrons. Where the energy comes from in Nuclear Fission? With the exception of hydrogen –which only contains a proton in its nucleus – all the elements are less massive than the mass of their constituent parts. For example helium contains four nucleon, 2 protons and 2 neutrons, in its nucleus. If you had added the mass of the individual nucleons together they would have more mass than a helium nucleus. So where has the mass gone? The missing mass has become the energy required to hold the nucleus together (the strong nuclear force) and this energy is known as the binding energy. The table above shows the amount of binding energy in each nucleon of each element. The higher binding energy per nucleon the more stable the nucleus of the element is and all elements want to be as stable as possible. As you can see from the table Iron is the most stable (has the most binding energy per nucleon) element and as such neither nuclear fusion nor fission is...
Binding Energy
Binding Energy is the energy required to break up the bonds of atoms or the energy released when small atoms combine. Binding Energy Nuclear Binding Energy Calculating Binding Energy Calculating the Assumed Mass Binding Energy per Nucleon Binding Energy This is the energy required to break up the bonds of atoms or the energy released when small atoms combine. There are two types of binding energy including electron binding energy (the energy required to overcome the electromagnetic force holding the electrons in orbit) and the Nuclear Binding Energy. Nuclear Binding Energy This can be thought of as either; the energy required to break up the bonds of the nucleus of an atom or the energy released when nucleons combine. The table above shows the amount of binding energy in each nucleon of each element. The higher binding energy per nucleon the more stable the nucleus of the element is and all elements want to be as stable as possible. As you can see from the table Iron is the most stable (has the most b-energy per nucleon) element and as such neither nuclear fusion nor fission is possible. As you can see from the table, all elements with less mass then iron will release energy through nuclear fusion as these element gain nuclear b-energy per nucleon as their mass increases. Elements with more mass than iron have less b-energy per nucleon and so they release energy from splitting into less massive elements through nuclear fission. Calculating Binding Energy A nucleus – except in the case of hydrogen...
Nuclear Fusion
Nuclear Fusion is the name given to the the event when the nuclei of two atoms combine creating a new heavier atomic nucleus. What is Nuclear Fusion? Overcoming the Electromagnetic Force Nuclear Fusion in Stars Energy Production through Nuclear Fusion What is Nuclear Fusion? Nuclear Fusion is a phenomenon where by the nucleus of two atoms fuse together creating a new heavier atomic nucleus. When two relatively small atomic nuclei have very high energies they are able to bond or fuse together making larger atoms. The atomic nuclei require a lot of energy to overcome the effects of the electromagnetic force before it is possible for nuclear fusion to occur. Overcoming the Electromagnetic Force Normally the electromagnetic force causes atomic nuclei to repel each other due to their positive charge, much like the same poles on a magnet. Electromagnetic force which causes the repulsive action quadruples as the distance between the two protons halves. This means that it takes a lot of energy to get these two protons, and two nuclei together. If they are able to get close enough another fundamental force, The Strong Force, comes into play and this force is much stronger than the electromagnetic force. It is the force that holds all nucleons together but they must be within one femtometres (the nucleus of an average atom is 4 femtometres in diameter). A = The Nucleus of the atom contains the protons and neutrons. Despite accounting for the majority of an atom’s mass, the nucleus procures a minute proportion of the total space. The Diameter of the nucleus is approximately 4 femtometres or 4...
Metis – Moon of Jupiter
Metis is one of Jupiter’s many moons, which is currently believed to have at least 63. It is one of four moons that make up the ‘Inner Group’. Where in the Solar System? The Inner Group Metis Where in the Solar System? Metis is one of Jupiter’s many moons, which is currently believed to have at least 63. It is one of four moons that make up the ‘Inner Group’. The Inner Group This is a group of four moons and as the name suggest they are situated closest to Jupiter at distances ranging from 128,000 – 200,000 km and taking between 7 and 16 hours to orbit the host planet. The Inner Group consists of Metis, Adrastea, Amalthea and Thebe, all of which are ‘irregular moon’ meaning they are not spherical due to their mass being too low (when cosmic bodies mass are large enough their gravity is able to shape the object into a sphere). Metis Metis is Jupiter’s closest moon, orbiting at around 128,000 km from the planet’s surface which is less than a third the distance of our own moon and earth (distance between earth and its moon is 384,400km). Metis got its name from the Greek mythical goddess of the same name, who was the first spouse of Zeus. It was discovered on the 4th of March 1979 by Stephen P Synnott, an American Astronomer and scientist working on NASA’s Voyager mission. Metis hurtles through space at an impressive 31.501 km/s (average speed) which is 70,465 miles per hour and takes...
Martian Moons – The Two Moons of Mars Phobos and Deimos
Martian Moons – The Two Moons of Mars Phobos and Deimos In the August of 1877 an American astronomer was looking at Mars in hopes of discovering a moon. He instead discovered two Martian Moons; Deimos and Phobos Phobos and Deimos—the mysterious moons of Mars—have intrigued scientists for generations. These tiny satellites orbiting the planet Mars offer clues to the planet’s past and its potential future in human space exploration. In this article, we dive deep into the Martian satellites Phobos and Deimos, unravel fascinating facts about Phobos, and learn how NASA science continues to probe their origins and significance. Whether you’re a space enthusiast, a student of astronomy, or just curious about what orbits the surface of Mars, this article will offer a thorough overview of the Mars’ moons, their orbits, potential missions, and why they’re crucial for future Mars exploration. Article Outline What Are the Moons of Mars and Why Are They Important? How Were Phobos and Deimos Discovered? What Are the Main Differences Between Phobos and Deimos? How Do the Martian Moons Orbit Mars? Could Phobos Crash Into Mars? What Has NASA Learned from Mars Missions? What Is the Origin of the Martian Moons? What Are the Plans for Future Moon Exploration? How Do Phobos and Deimos Compare to Earth’s Moon? Why Is Phobos Key to a Mars Sample Return Mission? Naming the Moons of Mars Shape and Orbit of the Martian Moons Origin of the Martian Moons Phobos Deimos Quick Comparison of Martian Moons What Are the Moons of Mars and Why Are They Important? Mars has two small moons, Phobos and Deimos, which are...
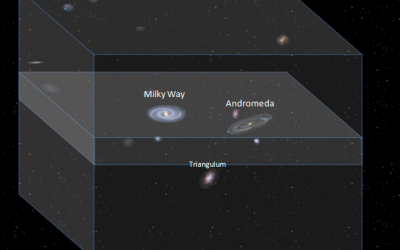
Local Galactic Group
The Local galactic group is the term we use for a group of over thirty galaxies in our local vicinity. What is our Local Galactic Group? Which Galaxies makeup our Local Galactic Group? Milky Way Group Andromeda The Triangulum spiral Galaxy (M33) Galaxies in our Local Group – Quick Reference What is our Local Galactic Group? Galaxies in a super cluster are not spread evenly but seem to be clumped together due to gravity into groups. The Local group is the term we use for a group of over thirty galaxies in our local vicinity (although various sources suggest more member galaxies these are not confirmed) and our group was first recognised by American astronomer and physicist Edwin Hubble. Which Galaxies makeup our Local Galactic Group? The two main Galaxies within our group are the Milky Way galaxy and the Andromeda galaxy (M31), each with their own surrounding group of smaller galaxies drawn in by the massive gravitational pull of these giants. Evidence suggests that these two very large galaxies were formed from absorption and culminations of smaller galaxies1. Observations of our two galaxies also suggest that they are being drawn closer together at a speed of 300km (or 180mi) a second and one day, in about several billion years from now, they too will merge as one super galaxy. This process will not happen quickly and it will take over about 2 billion years2 for the unity to be complete. Another smaller but still very large galaxy (third largest of the group but about...
Decay Chains
Decay Chains is the name given to the stages of radioactive decay of unstable isotopes or elements. Some radioactive isotopes decay into stable isotopes directly but some unstable or radioactive isotopes decay into other unstable isotopes many times before becoming stable and these stages are explained in the ‘decay chain’. These decay chains can be useful when radioactively dating samples by comparing the ratios of the isotopes in the chain. Decay Chain of Uranium-238 Below is the decay chain of Uranium-238 which is the most common isotope on earth. The stages shown in the table are the most likely outcome of radioactive decay naturally however at some stages it is possible for some of the elements to succumb to either alpha or beta decay which obviously changes the outcome. The possible variations and their likely hood and are shown in the key below. Stage Symbol Element Radiation Half-Life Decay Product 1 U-238 Uranium-238 alpha 4,460,000,000 years Th-234 (92U238 = 2He4 + 90Th234) 2 Th-234 Thorium-234 beta (β) 24.1 days Pa-234 (90Th234 = 91Pa234 + 0e-1) (see #1) 3 Pa-234 Protactinium-234 beta (β) 1.17 minutes U-234 (91Pa234 = 92U234 + 0e-1) (see #1) 4 U-234 Uranium-234 alpha 247,000 years Th-230 (92U234 = 90Th230 + 2He4) 5 Th-230 Thorium-230 alpha 80,000 years Ra-226 (90Th230 = 88Ra226 + 2He4) 6 Ra-226 Radium-226 alpha 1,602 years Rn-222 (88Ra226 = 86Ra222 + 2He4) 7 Rn-222 Radon-222 alpha 3.82 days Po-218 (86Ra222 = 84Po218 + 2He4) 8 Po-218 Polonium-218 alpha 3.05 minutes Pb-214 (84Po218 = 82Pb214 + 2He4) (see #2) 9 Pb-214 Lead-214 beta (β) 27 minutes Bi-214 (82Pb214 = 83Bi214...